Abstract
Abstract 3216
Poster Board III-153
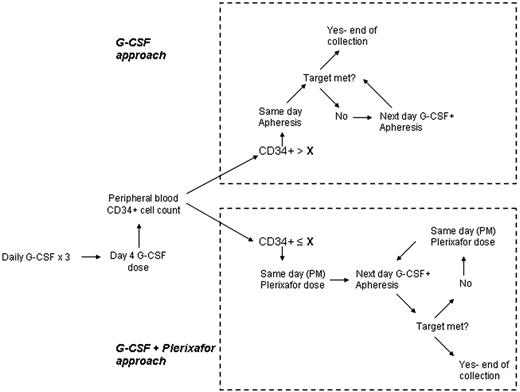
Plerixafor, an inhibitor of CXCR-4/SDF-1 binding, is approved for mobilization of peripheral blood hematopoietic stem cells (PBHSC) prior to autologous hematopoietic stem cells transplantation (AHSCT) for non-Hodgkin lymphomas and multiple myeloma (MM). In this context, plerixafor decreases the necessary number of days of apheresis and increases the proportion of patients reaching a target CD34+ cell count in the final product. Plerixafor, however, is expensive and not necessary in all patients undergoing PBHSC mobilization. We developed a cost-saving decision making algorithm that utilizes the CD34+ count in the peripheral blood on the 4th day of G-CSF administration (PB-CD34+) and the target CD34+ count for the specific patient (T-CD34+) to decide between starting collection on day 4 and continuing G-CSF administration only (G approach) or adding Plerixafor the night before each apheresis session and starting apheresis on day 5 (G+P approach) (Figure 1).
We reviewed mobilization and collection data in 50 consecutive mobilizations without the use of plerixafor in lymphoma and MM patients from our program to establish a mathematical correlation between PB-CD34+ and the average CD34+ count in the daily apheresis product (AP-CD34+). Based on available literature, we estimated a 3 fold increase in AP-CD34+ on each day of apheresis (starting on day 5) for a given PB-CD34+ with the addition of daily plerixafor. Reoccurring charges associated with mobilization and collection were based on charge for daily G-CSF administration (G) of $ 768.90, daily Plerixafor administration (P) of $ 7812.00, and average daily charges for apheresis (A) of $ 6922.00. The projected number of days of apheresis for each approach (NG and NG+P) was estimated by rounding up to the next natural number the ratio between T-CD34+and AP-CD34+. Total charges with G approach was estimated to be = (3+ NG) x G + NG x A and with G+P approach = (4+ NG+P) x G + NG+P x A + NG+P x P.
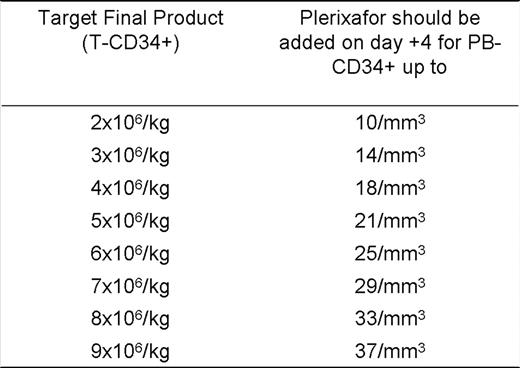
The projected AP-CD34+ with the G approach was found to be = (PB-CD34+ x 131,857 – 389,334) (R2 = 0.899). Consequently, the projected AP-CD34+ with the G+P approach was = 3 x (PB-CD34+ x 131,857 – 389,334). As expected, the G approach was more cost effective with high PB-CD34+. However, the G+P approach was more cost effective with low PB-CD34+ and it was possible to determine, for each T-CD34+, a maximum PB-CD34+ for which the G+P approach is cost effective (Table 1). This algorithm is being validated in our practice. After inclusion of 19 patients in the validation cohort (mostly patients at high risk for mobilization failure, median PB-CD34+ = 12), 6 (31.6%) patients completed collections with the G approach and 13 (68.4%) with the G+P approach. None of the patients have required more apheresis sessions than projected by this algorithm to meet the pre specified T-CD34+. Validation will continue at an approximate rate of 5 mobilizations/month and final results will be presented at the meeting.
A simple cost-saving algorithm was developed to support the decision on the fourth day of G-CSF mobilization to proceed with daily G-CSF and apheresis or to add daily plerixafor, based solely on collection target and peripheral blood CD34+ count. Preliminary validation data endorses the accuracy of this algorithm.
Fouts:Genzime: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal