Abstract
Abstract 3015
Poster Board II-991
PNH is a hematopoietic stem cell disorder in which unregulated activation of terminal complement leads to impaired quality of life and significant ischemic morbidities with shortened lifespan. Life-threatening thromboembolism (TE) is the most feared complication of PNH, accounting for 45% of patient deaths. Thrombosis has been observed in PNH patients regardless of the level of hemolysis. Additionally, platelet activation with subsequent consumption and thrombocytopenia are observed more often in PNH patients at risk for thrombosis.
Current laboratory PNH diagnostic methods rely on flow cytometry to characterize PNH clones. PNH granulocytes (Gran) are typically detected using antibodies to a variety of GPI-linked markers including CD55, CD59, CD16, CD24, and CD66b. Recently, FLAER, a fluorescent proaerolysin variant that binds directly to the GPI anchor, has been used to identify and quantify GPI-deficient WBCs at a very high level of sensitivity. Although these markers are well established to detect granulocytes with normal expression of GPI proteins (Type I cells) and complete loss of GPI proteins (Type III cells), less is known about their ability to detect granulocytes with partial loss of GPI proteins (Type II cells). The ability to detect both PNH Type II RBCs and WBCs would provide clinically important information since quantitation of only PNH RBC clones can be confounded by transfusion or hemolysis.
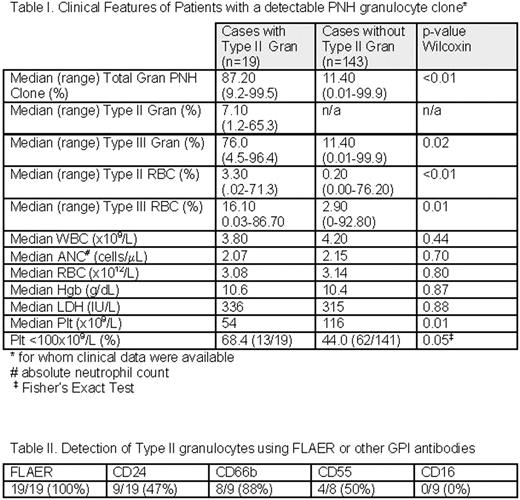
We evaluated 2,921 consecutive patient peripheral blood samples submitted for PNH diagnostic testing with a high-sensitivity flow cytometry assay for granulocytes that includes the fluorescent proaerolysin variant (FLAER) with confirmatory lineage-specific antibodies to GPI-linked antigens to distinguish Type I, II and III Gran clones. In addition, standard CD235/CD59 analysis was performed on the RBCs and evaluation with FLAER, CD14 and lineage-specific antibodies was performed on the monocytes. 216 patient samples (7.4%) had a detectable PNH gran clone (≥ 0.01% PNH Type III granulocytes and an absolute count of at least 50 cells). Clinical information was available for 162 of these patients (Table I). Of these samples, nineteen (8.8 %) patients demonstrated a distinct Type II Gran population, ranging in size from 1.2 – 65% (median clone size = 7%). In 4/19 patients, this Type II Gran population represented >50% of the total (Type II + Type III) PNH cells. In 10/19 patients (53%), a type II monocyte population was identified. Evaluation of the granulocyte markers (Table II) showed that the type II gran population was detectable in all cases by FLAER and in decreasing percentage by CD66b (88%), CD55 (50%), CD24 (47%) and CD16 (0%). Patients with Type II Gran clones had a significantly larger median total Gran PNH clone size than those without Type II Gran clones (87% vs. 11%; p= 0.0003), as well as larger median Type II and Type III RBC clones, likely a reflection of the ability to detect type II gran PNH clones with overall larger PNH clone sizes. Patients with Type II Gran clones showed significantly lower median platelet (plt) counts (54 ×109/L) than patients without Type II Gran clones (116×109/L; p< 0.01). Patients with Type II Gran clones had similar peripheral WBC, peripheral RBC, absolute neutrophil count, and hemoglobin (Hgb) compared to patients without Type II Gran clones, suggesting that differences in platelet counts are likely not due to differences in underlying marrow blood cell production.
Type II PNH cells are an important component of the PNH diagnostic evaluation and both RBC and Gran Type II clones should be enumerated. In a large population of patients tested for the presence of PNH clones using a high sensitivity flow cytometry assay, a significant proportion of patients were identified with Type II PNH Gran clones. This study identified FLAER as the best reagent to identify type II Gran PNH clones and showed CD16 was least useful. This study also identified a clinical association between the presence of significant Type II clones and thrombocytopenia, potentially indicative of terminal complement-mediated platelet consumption. These findings are consistent with an increased risk of thrombosis in patients with significant Type II PNH clones.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal