Abstract
Abstract 2844
Poster Board II-820
Skeletal complications including bone fracture, bone pain and hypercalcemia are major clinical events in patients of multiple myeloma (MM). Osteoclastgenesis is known to be induced by free receptor activator of nuclear factor kappa β ligand (RANKL) and inhibited by dimerization of RANKL and osteoprotegerin (OPG). OPG is also known as a soluble inhibitor of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL); therefore, a possible role of TRAIL as an osteoclast inducer is suggested, although the association of TRAIL with bone lesions in MM is a matter of debate. We thus investigated the expression of TRAIL mRNA in purified MM cells and analyzed its association with skeletal-related events.
MM cells were purified from bone marrow samples from 40 MM patients by CD138-immunomagnetic beads (Miltenyi Biotech, Paris, France). TRAIL mRNA expression in purified MM cells was analyzed by real time PCR(ABI PRISM 7700 Sequence Detector, Applied Biosystems). Simultaneous analysis of serum TRAIL concentrations, analyzed by ELISA (Diaclone, Cedex, France), and TRAIL mRNA-expression levels was also performed in 23 cases. Each of these patients was given a score called skeletal-related event score (SRE score) according to the skeletal complications (pathological fracture, bone-associated plasmacytoma, >12mg/dL hypercalcemia, and receiving an pathological fracture-related operation or radiation therapy).
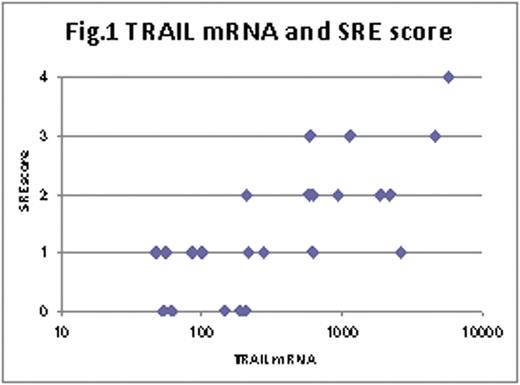
Significant association (p=0.0006) was seen between TRAIL mRNA expression levels and the SRE score (Fig.1). Serum calcium levels also had significant association to TRAIL mRNA expression levels (p=0.0050). On the other hand, no association of TRAIL mRNA with hemoglobin (p=0.3970) and platelets (p=0.9401) was seen. Serum TRAIL concentrations in MM cases, which were equivalent to those in healthy individuals, did not correlate to TRAIL mRNA expression levels in purified MM cells (p=0.4094).
The data suggest that MM patients with high TRAIL expression in MM cells tend to have more skeletal complications, which may be mediated by increased osteoclastgenesis. Since serum TRAIL concentrations did not correlate with TRAIL mRNA levels in MM cells, increased TRAIL expression in bone marrow microenvironment could be important. Despite of previous reports suggesting TRAIL-induced apoptosis of hematopoietic cells, the observed high TRAIL expression did not correlate with anemia or thrombocytopenia in our cases. Although mechanisms regulating TRAIL expression in MM cells and protection from TRAIL-induced apoptosis remain to be determined, our findings may introduce a new strategy targeting TRAIL to reduce skeletal events in MM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal