Abstract
Abstract 2707
Poster Board II-683
Rituximab has had a major impact on the treatment of B cell malignancies. The mechanisms responsible for mediating the anti-tumor effects of rituximab are complex. For example, complement can have both positive and negative effects on the ability of rituximab to induce target cell lysis. In particular, we recently reported that rituximab-mediated complement activation results in C3b deposition on the rituximab Fc. C3b then impedes interaction between rituximab and NK cell CD16, thereby limiting NK cell activation and ADCC.
GA101 is a type II anti-CD20 monoclonal antibody that mediates enhanced direct cell death induction. It has significantly reduced CDC activity compared to type I anti-CD20 antibodies such as rituximab. In addition, GA101 was engineered to mediate increased ADCC (Umana et al., ASH 2007). The current studies were designed to assess whether the decreased ability of GA101 to activate complement results in an enhanced ability of GA101 to activate NK cells when complement is present.
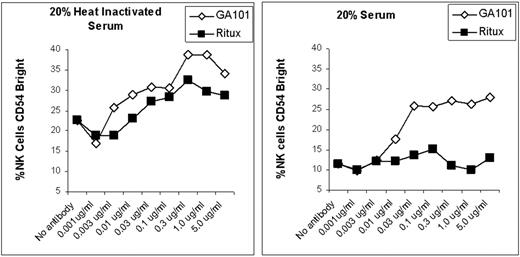
Peripheral blood mononuclear cells (PBMCs) were obtained from normal donors and added to Raji cells (Burkitt lymphoma cell line) at a 1:1 ratio. Various concentrations of rituximab or GA101 were added along with media, 20% autologous serum or 20% heat-inactivated autologous serum (heated to 57°C for 30 minutes). Samples were cultured for 20 hours. NK cell (CD3−, CD56+) activation, as determined by phenotypic changes, was evaluated by flow cytometry based on prior studies demonstrating that downmodulation of CD16, and upregulation of CD54 and CD69 are reproducible surrogates for mAb-induced NK activation and ADCC. Raji cells coated with either rituximab or GA101 were able to activate NK cells when cultures were performed in media alone or with heat-inactivated serum (left panel). In contrast, serum blocked the ability of rituximab to activate NK cells, but not the ability of GA101 to activate NK cells (right panel). Similar results were found when upregulation of CD69 or downmodulation of CD16 were evaluated as markers of NK activation and using PBMCs from two other donors.
We conclude that the presence of complement does not limit the ability of GA101-coated target B cells to activate NK cells. This is in contrast to rituximab-coated target B cells which are unable to activate NK cells in the presence of serum. These results suggest that the decreased ability of GA101 to fix complement could, paradoxically, enhance the efficacy of GA101 by resulting in enhanced activation of NK cells and increased ADCC.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal