Abstract
Abstract 2664
Poster Board II-640
Adoptive transfer of activated autologous or allogeneic natural killer (NK) cells has emerged as a safe and potentially efficacious immunotherapy for cancer. Alloreactive HLA haploidentical NK cells in the HSCT setting have been reported to enhance engraftment, reduce GVHD and prevent relapse of leukemia. The main restrictions to developing NK cells for immunotherapy are the limited quantity of cells available for adoptive transfer and the modulation of NK cell receptors and/or NK cell ligands as a recognized mechanism of immune escape in oncogenesis. To address the first hurdle, we have developed an efficient method to expand CD3−CD56+ primary NK cells in vitro using K562 artificial APCs expressing membrane-bound IL-21. Altering the balance of activating and inhibitory signals with immunosensitizing drugs is an attractive approach to addressing the second hurdle of NK cell therapy. DNA methylation inhibitors such as 5-Aza-2'-deoxycytidine and 5-azacytidine are being tested for their activity in AML, and have been shown to sensitize AML blasts to lysis by NK cells. However, there is little information on the direct immunologic effect of these hypomethylating agents on NK cell function, reconstitution, and phenotype.
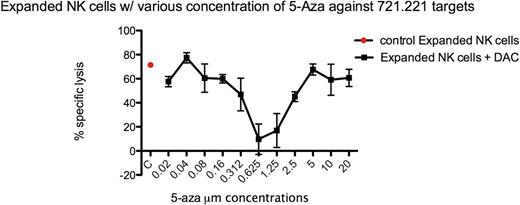
Using the calcein release assay, we investigated NK cell survival and lytic function against the NK cell-sensitive HLAnull 721.221 cells after exposing expanded NK cells to 5-Aza-2'-deoxycytidine. Human peripheral blood NK cells that had been expanded ex vivo in our laboratory were incubated with a wide range of 5-Aza-2'-deoxycytidine concentrations for 3 days in the presence of 50 IU/mL IL-2. Cell viability was tested every 24 h.
We found that 5-Aza-2'-deoxycytidine at higher concentrations resulted in reduced NK cell viability, but at lower concentrations inhibited NK cell killing. At these lower doses, NK cell inhibition was not associated with loss of viability, resulting in an overall U-shaped dose response curve to 5-Aza-2'-deoxycytidine with maximum inhibition in the 0.3 – 2.5 micromolar range.
This biphasic response to 5-Aza-2'-deoxycytidine matches that previously described for malignant cells in which reduced proliferation at higher concentrations resulted in reduced demethylation inhibition (Qin et al, Clin Cancer Res. 2007 Jul 15;13(14):4225–32). To assess the role of demethylation in NK cells in a more dynamic setting, we are investigating 5-Aza-2'-deoxycytidine effects on NK cell proliferation and phenotype during ex vivo expansion, and assessing NK cell recovery, phenotype, and function in a prospective cross-over trial of patients receiving 5-azacytidine post HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal