Abstract
Abstract 2537
Poster Board II-514
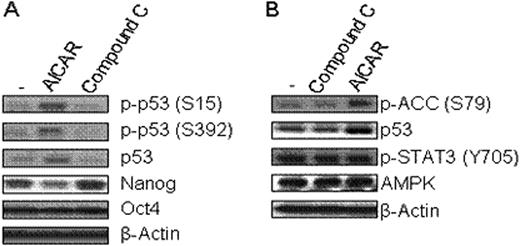
High glucose level is usually required for embryonic stem cell (ESC) culture but culture medium without glucose is a rather favorable condition to enhance differentiation of ESCs. However, the exact molecular relationship of how glucose metabolism affects ESC pluripotency remains unclear. AMP-activated protein kinase (AMPK), a key regulator for controlling the metabolism of glucose and fatty acids, is activated in response to a high intracellular AMP to ATP ratio (ATP-exhausting stress). We hypothesized that AMPK activity is associated with maintenance of ESC pluripotency and tested this hypothesis utilizing aminoimidazole carboxamide ribonucleotide (AICAR) and Compound C, respectively as an activator and a selective inhibitor of AMPK. Here, we demonstrate that in the mouse ESC line, R1, in the presence of LIF, AICAR significantly reduced protein expression of Nanog and SSEA-1 (Nanog: 365+/−21 vs. 270+/−25; SSEA-1: 13195+/−707 vs. 7572+/−507, mean fluorescence intensity (MFI), +/-SD, control vs. AICAR (0.5 mM, 24h treatment), n=3 expts, p<0.01). In contrast, Compound C enhanced protein expression of SSEA-1 and Nanog (Nanog: 365+/−21 vs. 555+/−35; SSEA-1: 13195+/−707 vs. 15864+/−894, mean fluorescence intensity (MFI), +/-SD, control vs. AICAR (0.005 mM, 24h treatment), n=3 expts, p<0.02). This indicates that AMPK has a positive effect on protein expression of Nanog and SSEA-1. We extended our study to investigate whether increased Nanog protein expression level via AMPK activity resulted from enhanced stabilization of Nanog protein by measuring the half-life of nanog protein following treatment of mouse ESCs with cycloheximide, a protein synthesis inhibitor. Nanog protein stability was not affected by AMPK activity. Next, we examined whether AMPK activator or inhibitor could change Nanog and Oct-4 mRNA levels. AICAR reduced Nanog mRNA expression by 40%, but did not influence Oct-4 mRNA expression. AMPK induces cell cycle arrest or apoptosis under ATP-exhausting stresses by phosphorylating and activating the p53 tumor suppressor, which could repress Nanog gene expression. Activated AMPK induced phosphorylation of p53 at serine 15 and 392 without disturbing LIF induced STAT3 signaling in this mouse ESC line (Fig. 1). Thus, we postulate that activated AMPK might inhibit Nanog gene activity by phosphorylation of p53. Taken together, our data suggest that AMPK activity in response to metabolic ATP and AMP levels may play a primary role in controlling ESC pluripotency through p53-mediated regulation of Nanog gene expression.
AMPK regulates Nanog expression. (A) R1 mouse ES cells were treated with AICAR (0.5mM) or Compound C (0.005mM) for 24h, and then cells were collected and lysed. (B) R1 cells were treated with AICAR or Compound C for 9h. The levels of indicated proteins were measured by western blotting.
AMPK regulates Nanog expression. (A) R1 mouse ES cells were treated with AICAR (0.5mM) or Compound C (0.005mM) for 24h, and then cells were collected and lysed. (B) R1 cells were treated with AICAR or Compound C for 9h. The levels of indicated proteins were measured by western blotting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal