Abstract
Abstract 2468
Poster Board II-445
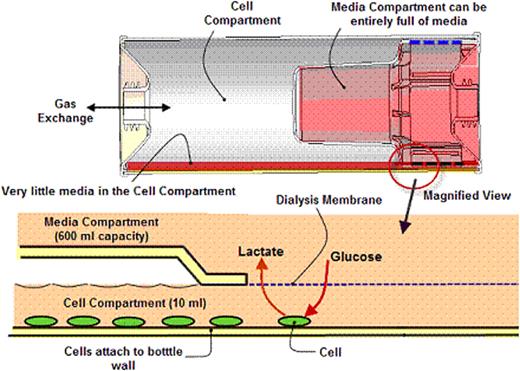
One impediment to the successful development of human therapies using genetically modified hemopoietic cells is the cost and complexity of vector manufacture, which limits the number of studies that can be accomplished within a reasonable time and cost. Retroviral transduction is a commonly used method for such ex vivo genetic modification and usually requires large volumes of retroviral supernatants, the titers of which are low compared with other viral vectors. We now describe the use of a novel culture device, called a Concentrated Retrovirus Expansion Device (CRED), which was developed by Wilson Wolf Manufacturing. This CRED is similar in external appearance and shape to other conventional roller bottles, and thus can be accommodated by standard roller racks. However the presence of an internal dialysis membrane allows the virus particles to become concentrated in the cell compartment, while the nutrient compartment is separated but easily accessible and can retain up to 250 ml of cell culture media without causing virus dilution. This CRED has a surface area of 600cm2 in the cell compartment, which, due to diffusion across the dialysis membrane, requires only 10 ml of media for cell survival, a tenfold lower requirement than for an equivalent area in a traditional cell factory or roller bottle.
To test the potency and stability of retroviral vectors produced in the CRED we cultured a clinical grade PG-13 producer cell line that produces the retroviral vector SFG-CAR-CD19/CD28/Z, which encodes a chimeric antigen receptor directed to the CD19 antigen (CD19-CAR). This vector is used in a current clinical protocol to transduce T cells for use in patients with advanced CD19+ B cell malignancies. We compared our standard procedure for retrovirus production (using a T175 flask) and 30 ml of IMDM against the CRED using 10ml of IMDM. Retroviral supernatant was harvested and frozen daily once cell confluence was attained (days 4-5 for the CRED and days 2-3 for the T175). Subsequently, to enumerate viable viral particles, 1×104 HeLa cells were seeded in a 6 well plate and after 24 hrs were transduced with the harvested retroviral supernatant using volumes ranging from 10-300ml in the presence of 1ml of Polybrene (10ug/ul Millipore). Six days later the cultures were evaluated by flow cytometric analysis to assess transduction efficiency, using a monoclonal antibody that recognized the CH2CH3 sequence of the CAR-CD19/CD28/Z transgenic protein. The transduction efficiency of virus harvested from the CRED was at least 4 times greater than that of the cells transduced with supernatant prepared using with the conventional method; 10ul (7.5%±4.5% vs 2.5%±0.28%), 20ul (11.9%±6.3% vs 4.9%±0.1%), 50ul (25.9%±7.4% vs 7.9%±0.9%), 100ul (45.2%±11.5% vs 9.3%±0.6%), 200ul (75.15%±15.6% vs 16.9%±1.5%), 300ul (85.7%±8.6% vs 21.4±1.8%) (n=4 independent experiments).
To ensure that the CRED-produced vector was stable we exposed the harvested supernatant to 6 consecutive freeze-thaw cycles, and compared titers before and after each. We initially obtained 86.8% transduction efficiency of HeLa cells that progressively but slowly declined after each cycle (82.4%, then 80.2%, 80%, 68.7%, 60.9% and 55.5%). Hence the CRED can produce large quantities of high titer retrovirus without media change. This concentrated virus is sufficiently stable to resist freeze thaw cycles and occupies 10 fold less storage space than conventional reagents. This combination of features should enable this scalable production technique to facilitate clinical studies of retroviral vectors.
Cross-sectional view of the CRED, demonstrating how cells can be cultured in a very small volume of media without losing the ability to receive a virtually unlimited supply of nutrients.
Cross-sectional view of the CRED, demonstrating how cells can be cultured in a very small volume of media without losing the ability to receive a virtually unlimited supply of nutrients.
Wilson:Wilson Wolf manufacturing: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal