Abstract
Abstract 2453
Poster Board II-430
NOD2 polymorphisms are independent risk factors for Crohn's disease and graft-versus-host disease (GVHD). In Crohn's disease, the pro-inflammatory state resulting from NOD2 mutations have been associated with a loss of anti-bacterial function of enterocytes, such as paneth cells. NOD2 has not been studied in experimental allogeneic bone marrow transplantation (allo-BMT).
We studied the role of NOD2 during inflammation in murine models of graft-versus-host disease (GVHD) and in experimental colitis. To investigate the role of NOD2 in regulating GVHD in allo-BMT recipients, we used MHC-matched as well as MHC-disparate allo-BMT models. We first assessed the role of NOD2 deficiency of the allo-BMT donor (either donor T cells or bone marrow) and found no significant impact on the development of GVHD. In contrast, we observed significantly more lethal GVHD in NOD2-/- allo-BMT recipients as compared with WT allo-BMT recipients. We next created chimeric mice, which were NOD2 deficient either in the hematopoietic or non-hematopoietic system. After three months, we performed allo-BMTs (B10BRþB6 and LPþB6) using either WT, NOD2-/- or chimeric recipients. We found that the NOD2 deficiency in the hematopoietic system of the recipient, as opposed to a NOD2 deficiency in the non-hematopoietic system, is responsible for the increased severity of GVHD (Fig. 1A). In NOD2-/- allo-BMT recipients we observed that the absolute number of donor T cells as well as their activation status was significantly increased. Next, we transferred CFSE labeled allogeneic WT T cells to NOD2-/- allo-BMT recipients and found increased proliferation and activation, suggesting that NOD2 plays a role in the regulation of host antigen presenting cells (APCs). We then quantified the expression of activation markers and co-stimulatory molecules on host dendritic cells (DCs): CD40, CD80 and CD86 were significantly up-regulated of on host NOD2-/- DCs as compared with WT DCs during GVHD. To study DC function we selected splenic DCs from WT and NOD2-/- allo-BMT recipients with GVHD and used them as stimulators in mixed leukocyte reactions (MLRs). NOD2-/- DCs had a significantly increased ability to induce proliferation of allogeneic T cells as compared with WT DCs. Finally, we used bone marrow chimeras in an experimental colitis model (which has not been done before) and observed again that NOD2 deficiency in the hematopoietic cells results in increased intestinal inflammation (Figure 1B). We conclude that NOD2 regulates the development of GVHD through its inhibitory effect on host APC function.
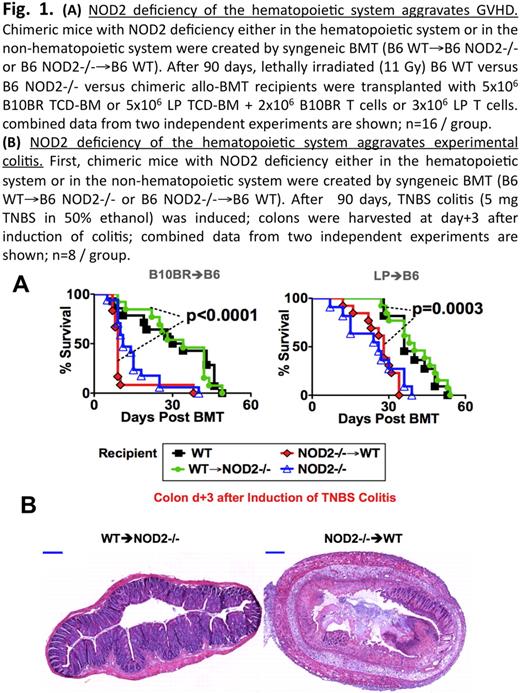
(A) NOD2 deficiency of the hematopoietic system aggravatges GVHD. Chemeric mice with NOD2 deficiency either in the hematopoietic system or in the non-hematopoietic system were created by syngeneic BMT (B6 WT → B6 NOD2-/- or B6 NOD2-/→ B6 WT). After 90 days, lethally irradiated (11 Gy) B6 WT versus B6 NOD2-/- versus chemeric allo-BMT recipients were transplanted with 5×106 B10BR TCD-BM or 5×106 LP TCD-BM + 2×106 B10BR T Cells or 3×106 LP T cells. combined date from two independent experiments are shown; n = 16/group. (B) NOD2 deficiency of the hematopoietic system aggravates experimental colitis. First, chemeric mice with NOD2 deficiency either in the hematopoietic system or in the non-hematopoietic system were created by syngeneic BMT (B6 WT→B6 NOD2-/- or B6 NOD2-/-→ B6 WT). After 90 days, TNBS colitis (5 mg TNBS in 50% ethanol) was induced; colons were harvested at day+3 after induction of colitis; combined data from two independent experiments are shown; n=8 / group.
(A) NOD2 deficiency of the hematopoietic system aggravatges GVHD. Chemeric mice with NOD2 deficiency either in the hematopoietic system or in the non-hematopoietic system were created by syngeneic BMT (B6 WT → B6 NOD2-/- or B6 NOD2-/→ B6 WT). After 90 days, lethally irradiated (11 Gy) B6 WT versus B6 NOD2-/- versus chemeric allo-BMT recipients were transplanted with 5×106 B10BR TCD-BM or 5×106 LP TCD-BM + 2×106 B10BR T Cells or 3×106 LP T cells. combined date from two independent experiments are shown; n = 16/group. (B) NOD2 deficiency of the hematopoietic system aggravates experimental colitis. First, chemeric mice with NOD2 deficiency either in the hematopoietic system or in the non-hematopoietic system were created by syngeneic BMT (B6 WT→B6 NOD2-/- or B6 NOD2-/-→ B6 WT). After 90 days, TNBS colitis (5 mg TNBS in 50% ethanol) was induced; colons were harvested at day+3 after induction of colitis; combined data from two independent experiments are shown; n=8 / group.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal