Abstract
Abstract 2447
Poster Board II-424
Allogeneic bone marrow transplantation (BMT) represents the most effective treatment for patients with high risk and relapsed hematologic malignancies. One of the major complications of allogeneic BMT is graft-versus-host disease (GvHD), which is caused by donor T cells reacting against host antigens. These same alloreactive donor T cells can provide a beneficial ¡°graft-versus-leukemia¡± (GvL) effect as well resulting in reduction in leukemia relapse. Because regulatory T cells (Tregs) have been shown to suppress GvHD while preserving GvL, their use in the allogeneic transplant setting provides a promising strategy to treat GvHD. However, three major obstacles prevent their routine use in human clinical trials: 1) the low circulating numbers of Tregs in peripheral blood, 2) loss of suppressor activity following ex vivo expansion and 3) the lack of Treg-specific markers to purify ex vivo expanded Tregs. Foxp3 is a forkhead transcription factor which is both exclusively expressed in Tregs and, when overexpressed in conventional effector T cells (Teff), can convert these Teff into functionally suppressive Treg-like T cells. The Foxp3 locus is unmethylated in Tregs while highly methylated and silenced in all other T cells. Several groups have shown that the hypomethylating agent azacitidine (AzaC) induces FOXP3 expression in non-Tregs. Furthermore, we have shown that treatment of anti-CD3/CD28 antibody-coated bead-activated CD4+CD25- T cells with AzaC results in robust and prolonged (>7 days) expression of FOXP3. AzaC-induced FOXP3 expression is also associated with a potent Treg-like suppressive phenotype in vitro. Thus, we hypothesize that AzaC treatment of mice after allogeneic BMT will dramatically mitigate GvHD while preserving GvL via transcriptional regulation of Foxp3 in alloreactive Tconv.
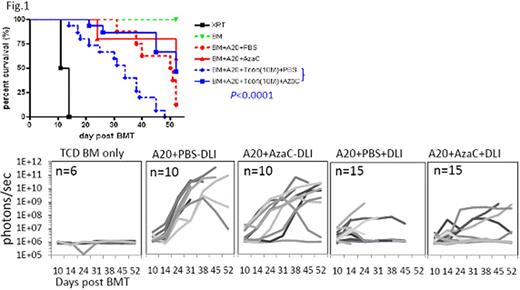
In murine T-cell depleted BMT model (B6¡æBalb/c; 900 cGy TBI) with delayed infusion of conventional T cells (Tconv) (2 ×106) at day 11 post BMT, followed by subcutaneous treatment of AzaC (2 mg/kg at days 15, 17, 19, and 21 post BMT), we found that AzaC dramatically suppressed GvHD caused by allogeneic donor T cells while maintaining donor (H2Kb) engraftment of all lineages. We found that the AzaC group had significantly higher FOXP3+ Tregs than in PBS control group and that these Tregs were derived from donor T cells, suggesting that the suppression of GvHD was mediated by AzaC-induced Tregs. We further tested whether AzaC treatment of mice transplanted with allogeneic T cells preserve GvL while mitigating GvHD. Using the same murine allogeneic BMT model, Click Beetle Red luciferase-expressing A20 leukemia cells (BALB/C derived) were injected with T-cell depleted BM and 10 × 106 Tconv and in vivo bioluminescence imaging was performed to assess tumor burden in vivo post transplant. We found that AzaC treatment mitigated GvHD without abrogating GvL (Fig. 1) or donor engraftment. Thus, the adminstration of hypomethlating agents like AzaC in vivo after allogeneic stem cell transplant dramatically reduces GvHD while maintaining both donor engraftment and a potent GvL effect providing the foundations for future clinical trials.
DiPersio:Genzyme Corporation: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal