Abstract
Abstract 2372
Poster Board II-349
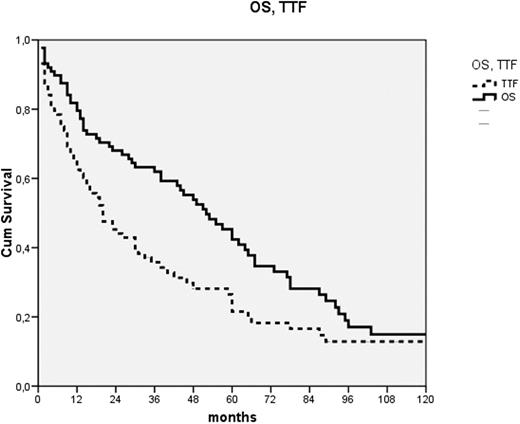
Chlorambucil is an effective and safe oral alkylating agent used for treatment of indolent lymphoproliferative disorders. While once considered a drug of choice for CLL, it has recently fallen out of favor, mostly due to clinical trials showing that fludarabine-based combinations have better disease-free, albeit not overall survival (OS). In these studies chlorambucil was administered once every 2-4 weeks. Results of some clinical trials suggest that continuous use of high doses of chlorambucil (≥ 10mg /day) is superior to intermittent administration. However, there are very few publications reporting outcomes of this type of treatment, especially outside of clinical trials. Here we summarize our experience with this approach in unselected patients with CLL. This was a retrospective study performed by chart-review of patients treated at our center. Patients were included in this analysis if they had CLL according to standard immunologic, morphologic and hematologic criteria, were in need of treatment and received chlorambucil at a daily dose of 10 mg or more for at least 4 weeks or until response or unacceptable toxicity as front-line treatment. Simultaneous administration of steroids but not other cytotoxic drugs was allowed. Use of low-dose chlorambucil maintenance (≤ 5 mg/day) after stopping high-dose chlorambucil was not considered treatment failure. The Kaplan-Meier method was used for creating survival curves and log-rank for comparisons of temporal variables. We identified 88 patients fulfilling the inclusion criteria, 70 men and 18 women, who were at the time of treatment start 38-88 years old (median 64). Nineteen patients were in Binet stage A, 38 B and 31 C; 2 were in Rai stage 0, 27 stage 1, 38 stage 2, 6 stage 3 and 25 stage 4. Twenty-nine had a low and 59 high total-tumor-mass index (TTM). The dose of chlorambucil ranged form 10 to 20 mg daily (median 15) and duration of treatment 2 to 20 wks (median 5). Forty-seven patients (53%) also received low-dose chlorambucil maintenance. The treatment was well tolerated. Three patients (3%) had serious infections, 2 died; 5 (6%) had severe hematological toxicity, mostly thrombocytopenia. Nausea, very common with pulse-doses of chlorambucil, was absent or mild; only 1 patient had severe nausea. Sixty-nine patients (78%) responded. After a median follow-up of survivors of 58 months, 26 patients are still alive and 17 have not failed treatment. Median OS was 53 months, 95% confidence-interval (CI) 40-66 months, time to treatment failure (TTF) 20 months (95% CI 13-27) (Fig. 1) and progression-free survival 15 months (95% CI 8-22). Age had no influence on treatment outcome. OS of patients with favorable stages (Rai 0-2, Binet A&B) was 60 months and of those with unfavorable stages 44 months. TTF was 30 and 11 months in these groups respectively. Both of these differences barely failed to reach statistical significance (p=0.067 and 0.058). TTM of 9 or higher was the only statistically significant negative prognostic factor that we were able to identify. OS was 78 vs. 44 months (p=0.021) and TTF 46 vs. 15 months (p=0.027). In conclusion, our results suggest that continuous administration of relatively high doses of chlorambucil is superior to intermittent administration, resulting in better efficacy and lower toxicity. While our results are inferior to those generally reported with fludarabine-based front-line treatments, continuous high-dose chlorambucil is less toxic, cheaper, the duration of treatment is shorter and OS is not different. This makes continuous high-dose chlorambucil an interesting approach for elderly, time- or financially constrained patients or health-care systems. Also, the low-toxicity and short treatment duration make it an interesting platform for the addition of non-toxic targeted drugs, like rituximab.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal