Abstract
Abstract 234
Thymic GVHD (tGVHD) after allogeneic bone marrow transplantation (allo-BMT) is associated with prolonged immunodeficiency. We have previously shown that thymic output after allo-BMT is directly related to thymus size, and inversely related to donor T cell dose and GVHD severity. Additionally, radiation-containing preparative regimens upregulate the death receptors Fas and DR5 on thymic stroma (especially epithelium) while decreasing expression of the anti-apoptotic protein cFLIP, thereby sensitizing the thymus to GVHD. Moreover, small numbers of donor alloreactive T cells are sufficient to cause tGVHD, and they utilize the Fas/Fas ligand (FasL) and TRAIL/DR5 pathways to mediate damage thymic stroma, architecture and function.
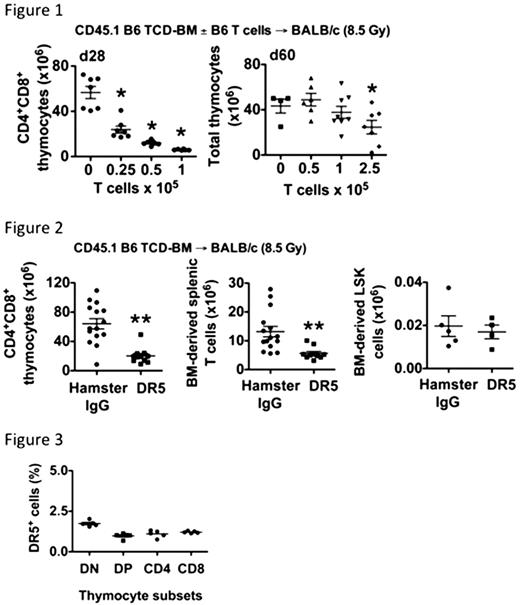
We performed experiments in both MHC-mismatched and MHC-matched minor antigen-disparate model systems, and demonstrated the exquisite sensitivity of the thymus to as few as 1–2.5×105 donor T cells, which mediated tGVHD without evidence of overt clinical disease or significant weight loss. Additionally, tGVHD is partially reversible in our model systems (contingent on a low T cell dose), such that mice with tGVHD exhibit a transient but partially reversible decrease in thymic cellularity when measured at days 28 vs. 60 post-transplant (Figure 1).
To further study the role of TRAIL in tGVHD, we asked whether (1) alloreactive T cells and (2) the inflammation associated with conditioning and acute GVHD, were strictly required for TRAIL/DR5-mediated thymic damage. We treated recipients of T cell-depleted allo-BMT with the amDR5-1 agonistic antibody (0.2 mg i.p. per dose) either in the ‘early‘ peri-transplant period, or ‘late,‘ in the second week post-transplant.
Allo-BMT recipients treated ‘early‘ with amDR5-1 had significantly decreased thymic cellularity and splenic BM-derived T cells as compared with controls. Furthermore, we observed similar BM cellularity and BM-derived lineage− sca-1+ckit+ (LSK), all in the absence of donor alloreactive T cells and GVHD (Figure 2). We observed similar results with mice treated ‘late‘ amDR5-1 using the later schedule, which indicates that the thymus has continued sensitivity to TRAIL throughout the post-transplant period, and that GVHD and/or conditioning-associated cytokines are not required to enable TRAIL-mediated damage to the thymus.
We further assessed the expression of DR5 on donor BM-derived thymocytes to determine whether amDR5-1 acted directly on thymocytes. We observed that on day 28 after T cell-depleted allo-BMT, only 1-2% of donor thymocytes expressed DR5, suggesting that amDR5-1 (and potentially TRAIL) mediate their effects on thymic cellularity and function primarily via an indirect mechanism (Figure 3).
These data suggest to us that significant damage to the thymus and thymopoiesis during allo-BMT:
can be mediated by soluble TRAIL agonists, even without the presence of whole donor alloreactive T cells
can occur, via the TRAIL pathway, without a full complement of the inflammatory processes typically associated with GVHD pathophysiology, including the cytokine storm, and
can occur early and late post-transplant, and that early exposure of the thymus to TRAIL/DR5 signals are sufficient to cause a durable loss in thymic cellularity, donor CD4+CD8+ thymocytes counts, and numbers of donor BM T cells exported to the periphery.
ccurs via the TRAIL pathway, without significant direct damage (via TRAIL/DR5 interactions) to the bone marrow compartment, and thus by implication, thymic-seeding precursors derived from the BM.
Together, our data in clinically-relevant mouse allo-BMT models suggests that the thymus is highly sensitive to GVHD and endures severe damage at relatively low levels of systemic GVHD. Moreover, post-transplant thymic atrophy is a partially-reversible process which depends on the T cell dose, and which occurs via the TRAIL pathway. Finally, we provide significant mechanistic insight which shows that TRAIL-mediated thymic damage can occur (1) throughout the early post-transplant period, and (2) does not strictly require alloreactive T cells, or the inflammatory processes associated with conditioning and GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal