Abstract
Abstract 2319
Poster Board II-296
Data from two phase 3 clinical trials indicate that plerixafor plus G-CSF is safe and more effective than G-CSF alone in mobilizing CD34+ hematopoietic stem cells (HSC) for autologous HSC transplantation (auto-HSCT) in patients with non-Hodgkin's lymphoma (NHL) or multiple myeloma (MM). Herein we report 1-year overall survival in patients with NHL or MM who have undergone auto-HSCT with cells mobilized with plerixafor plus G-CSF compared to placebo plus G-CSF. Furthermore, we also report 1-year overall survival in patients with NHL who failed mobilization on either arm and entered a rescue protocol.
Data were obtained from two prospective, randomized, double-blind, placebo-controlled, phase 3 clinical trials that compared the safety and efficacy of plerixafor (0.24 mg/kg/day SQ) plus G-CSF (10 mg/kg/day) with placebo plus G-CSF for mobilization of HSC for autologous HSCT in patients with NHL or MM. In the NHL study, patients in either study arm who failed mobilization (&0.8 ×106 CD34+ cells/kg in 2 days or &2 × 106 CD34+ cells/kg in 4 days) were eligible to enter the rescue protocol and received plerixafor + G-CSF mobilization. Overall survival in patients was estimated by the Kaplan-Meier method.
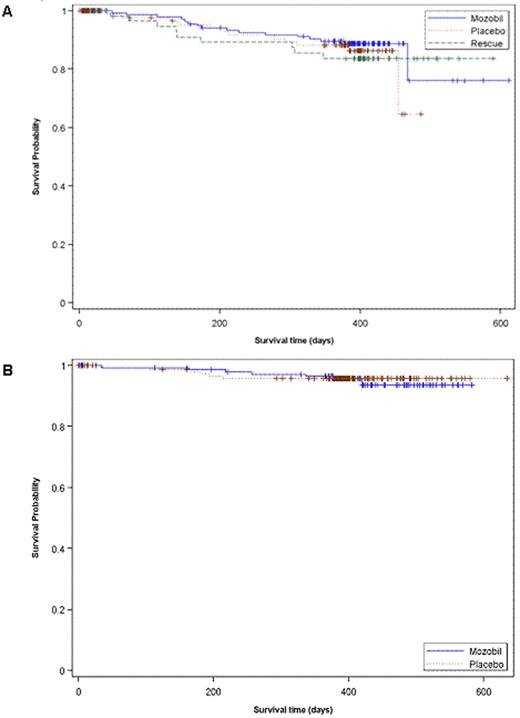
In the NHL study, 135/150 (90.0%) patients in the plerixafor group compared with 82/148 (55.4%) patients in the placebo group, underwent transplantation (p&0.001) with a median (range) of 5.41 (1.95-17.6) and 3.85 (1.98-8.74) × 106 CD34+ cells/kg, respectively (p&0.001). A total of 62 patients (10 from the plerixafor arm and 52 from the placebo arm) failed mobilization and proceeded to rescue mobilization with plerixafor + G-CSF. Of these, 52 patients (84%) proceeded to transplantation with a median (range) of 3.8 (1.1-10.5) x 106 CD34+ cells/kg. Kaplan-Meier estimates of 1-year overall survival for patients in the plerixafor and placebo groups in the primary ITT population (rescue patients were censored as of consent date for rescue) were 76.0% and 64.7%, respectively. Kaplan-Meier estimate of 1-year overall survival in the rescue group was 83.7%. The Log rank p-value comparing all 3 survival estimates (plerixafor, placebo and rescue) was 0.765 (Figure 1A). In the MM study, 142/148 (95.9%) patients in the plerixafor group and 136/154 (88.3%) patients in the placebo group underwent transplantation (p=0.014) with a median (range) of 5.40 (1.20-16.74) and 3.96 (1.76-16.88) × 106 CD34+ cells/kg, respectively (p&0.001). In this trial, Kaplan-Meier estimates of 1-year overall survival in the plerixafor and placebo groups were 93.6% and 95.8%, respectively (Log-rank p-value=0.771; Figure 1B).
These data suggest that overall survival at 1 year is similar between patients with MM or NHL mobilized with plerixafor plus G-CSF compared to placebo plus G-CSF. Furthermore, in the NHL study, patient survival in the rescue protocol was similar to that seen in the phase III trial. Collection of follow-up data on overall survival is ongoing.
Kaplan Meier Curve for One Year Overall Survival in A) NHL Patients (3101 Study) - Primary ITT and Rescue and B) MM Patients (3102 Study). Survival time depicted in days post randomization of study treatment
Kaplan Meier Curve for One Year Overall Survival in A) NHL Patients (3101 Study) - Primary ITT and Rescue and B) MM Patients (3102 Study). Survival time depicted in days post randomization of study treatment
Micallef:Genzyme Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding. Stiff:Genzyme Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Stadtmauer:Genzyme Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Bolwell:Genzyme Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Marulkar:Genzyme Corporation: Employment, Equity Ownership. Hsu:Genzyme Corporation: Employment, Equity Ownership. DiPersio:Genzyme: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal