Abstract
Abstract 23
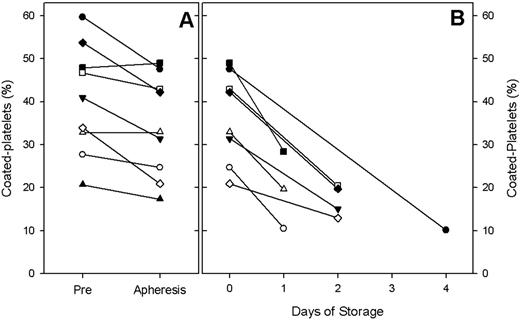
Platelets initiate hemostasis by adhering to the site of vascular injury and providing a negatively charged surface for thrombin generation. A subset of platelets, referred to as coated-platelets, has an enhanced ability to generate thrombin. Coated-platelets, observed in vitro upon dual agonist stimulation with collagen and thrombin, retain several serotonin-derivatized proteins on their surface and express phosphotidylserine, creating a highly active prothrombinase complex. Experimental studies have concluded that dogs deficient in coated-platelets manifest with excessive nasal, intraocular and post-operative bleeding, suggesting a key role for coated-platelets in hemostasis. The fraction of coated-platelets made by normal individuals is approximately 30% with a range of 15% – 55%. Platelet concentrates are stored at room temperature to preserve their viability and must be transfused within five days of collection secondary to a high risk of contamination. We conducted a pilot study in healthy platelet donors to evaluate how the process of apheresis and in vitro storage affect the ability to generate coated-platelets. Secondarily, we aimed to evaluate the coated-platelet potential of apheresis platelet units transfused in clinical practice. Methods: Healthy voluntary platelet donors presenting at the Oklahoma Blood Institute were enrolled and demographic data such as age, sex, smoking status, donation history, past medical history and medications were noted. A Pre-donation blood sample was obtained. Thereafter, donors underwent apheresis using the Caridian BCT Trima system via continuous centrifugation in a closed system per guidelines outlined by the American Association of Blood Banks (AABB). A Post-donation sample was drawn from the platelet concentrate bag 30 minutes after apheresis. Units were packaged, processed and stored per routine procedures. A third platelet sample was obtained when the unit was released for transfusion. Platelet rich plasma was prepared and coated-platelets were assayed as previously described (J. Thromb. Haemostasis 6:609, 2008). Statistical analysis was performed using paired t-test to compare the change in percentage of coated-platelets between the three samples. Results: Nine donors were enrolled. The mean percentage of coated-platelets in the Pre-donation samples was 40.4% (20.7%–59.7%). Thirty minutes after the apheresis collection procedure, the mean percentage of coated-platelets in the concentrate was 34.3% (17.3%–49.0%) suggesting a 15% decline in percentage of coated-platelets with the process of apheresis (p<0.01). Eight units were transfused within one to four days of collection. The mean percentage of coated-platelets in the transfused product was 17.1 % (10.1%–28.4%); a 52% decline in the percentage of coated-platelets during the process of storage was noted (p<0.001). [Figure A and B].
Coated-platelets are a recently recognized component of hemostasis, and the ability of stored platelets to retain coated-platelet potential is unknown. Our preliminary results indicate that the process of platelet apheresis and storage significantly decreases the ability to generate coated-platelets. Current quality control guidelines for platelet storage measure platelet viability with factors such as pH, pCO2, pO2, lactate and platelet morphology; however, there is no evaluation of the hemostatic function of stored platelets relative to coated-platelets. Our results reveal that during storage following current transfusion guidelines, the percentage of coated-platelets decreases. Part of this decline appears to be associated with the apheresis process itself and further progresses with storage. Our study provides the first evidence that retention of coated platelet levels during preparation and storage is problematic and may be an important factor in evaluating the hemostatic potential of infused platelets.
No relevant conflicts of interest to declare.
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal