Abstract
Abstract 2197
Poster Board II-174
Imatinib is very effective for patients with CML in chronic phase (CP). While some patients (pts) may fail therapy with imatinib (because of resistance or intolerance), effective salvage therapy is available with new generation TKI (dasatinib, nilotinib, bosutinib). Currently the efficacy of each therapy is judged by their individual impact on overall survival (OS) and event free survival (EFS). However calculations of EFS for a patient does not take into account the effect of successful salvage therapy with subsequent TKI. We therefore studied the current leukemia free survival (CLFS) to obtain a more accurate impression of the outcome of pts with CML treated with sequential TKI.
To provide an accurate estimation of long-term outcome with TKI therapy among pts with CML treated with sequential TKI.
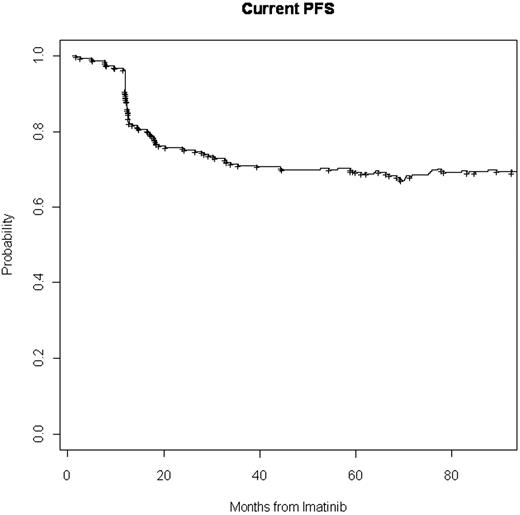
All pts with CP CML who received imatinib after failure to interferon-alpha (IFN) therapy at MD Anderson Cancer Center (MDACC) were included n this analysis. Events were defined as failure to achieve a complete cytogenetic response (CCyR) by 12 months of therapy, loss of CCyR, or discontinuation of therapy because of toxicity or any other causes after 18 months without achieving CCyR. Pts who failed imatinib either went to a 2nd TKI or other class of therapy. EFS is defined as survival without evidence of relapse at anytime after imatinib while CLFS is defined as survival without evidence of leukemia relapse at the time of most recent assessment. Thus, a loss of response would count as an event, but a response to salvage therapy would “repair” that event. Pts receiving any therapy other than a TKI after imatinib or other TKI is counted as an irreparable failure.
Three hundred and five pts were treated at MDACC with imatinib after IFN failure. The median age was 54 years (range 23-81), 169 (55%) were males. Median time on IFN was 1.5 yrs (range 0.1-10 yrs). Best response to IFN was MCyR in 114 (37%), minor cytogenetic response in 35 (11%), and CHR in 86 (28%). Among the others, 15 were intolerant to IFN, 29 pts were resistant and 26 pts had an unknown response. Reasons for being off IFN included 141 (46%) cytogenetics .{/MAIN;137}resistance, 37 (12%) hematological resistance, 123 (40%) intolerance, and 4 (1%) unknown. On imatinib 201 (66%) pts achieved CCyR; 64 of them discontinued imatinib in CCyR because of toxicity or other reasons, none of 64 pts received a 3rd line TKI. 27 (9%) pts who failed imatinib were treated with another TKI and attained CCyR. Of them, 18 remained in CCyR, 7 eventually lost CCyR, and 2 lost CCyR but attained and maintained CCyR with a 3rd TKI. Seventy-seven (25%) pts failed imatinib and could not be salvaged either due to unavailability of a 2nd TKI or failure to achieve CCyR with a 2nd TKI. EFS for this patient population is 63% at 7 years, whereas the CLFS is 70%.
CLFS can measure the actual benefit of TKI therapy by accounting for failures and subsequent responses. Despite the unavailability of 2nd generation TKI during the early part of the observation period, the CLFS of 71% at 7 years demonstrates the extraordinary benefit obtained with these agents alone or, when needed, in sequence.
Kantarjian:BMS: Research Funding; Novartis: Research Funding. Cortes:Novartis: Research Funding; BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal