Abstract
Abstract 2076
Poster Board II-53
Recently, further refinement of the “unfavorable” cytogenetic category in AML demonstrated that a “monosomal karyotype” (MK+) – defined as two or more distinct autosomal chromosome monosomies or one single autosomal monosomy in the presence of structural abnormalities – carries a markedly inferior prognosis to that of a “non-monosomal karyotype” (MK-) – defined as a group with various non-core binding factor (CBF) cytogenetic abnormalities but who are MK negative (J. Clin Onc 26:4791). This finding was based on data from patients predominantly with de novo AML. We analysed data from a phase II trial of patients with sAML to examine whether the monosomal karyotype is also predictive of poor outcome in sAML patients.
The karyotypes of pre-treatment blast cells from previously untreated sAML patients on a phase II study were reviewed to identify categories of core binding factor karyotype (CBF), normal karyotype (CN), MK+ and MK- (as defined above), and compared to clinical outcomes. Patients on the study all received induction therapy with amonafide 600 mg/m2/day IV days 1-5 and cytarabine 200 mg/m2/day IV days 1-7. Consolidation consisted of stem cell transplant (HSCT, n=10) or intermediate-dose (IDAC, n=13)/high-dose (HiDAC, n=7) cytarabine, depending on age and available HSCT donors.
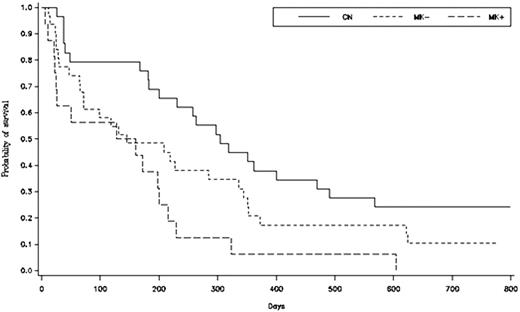
88 patients were treated. 2/88 (2%) were CBF, 29/88 (33%) were CN, 16/88 (18%) were MK+ and 31/88 (35%) were MK-. Patients with CBF and the 10 patients with unknown karyotype at baseline were excluded from the analysis. CR+CRi rate by karyotypic group was 19/29 (66%) for CN, 11/31 (35%) for MK-, 2/16 (13%) for MK+ (CN vs MK- p<0.05; CN vs MK+ p<0.05; MK- vs MK+ p=0.09). Median duration of remission was 282 days for CN, 304 days for MK-, and not reached for the 2 MK+ patients who achieved CR (p=NS). Median overall survival: 304 days CN, 145 days MK- and 144 days MK+ (Log rank test for survival: CN vs MK- p=0.07; CN vs MK+ p<0.05; MK- vs MK+ p=0.06).
In this study of 88 patients with secondary AML, a monosomal karyotype was predictive of inferior response to therapy compared with both a normal karyotype and a non-monosomal “unfavorable” karyotype. Importantly, the 2-year overall survival in the MK+ group was significantly inferior to that in the CN group. Of note durable complete remissions were observed in responders regardless of karyotype. Though the number of patients in this study is relatively small, to our knowledge this is the only prospectively defined study of sAML patients demonstrating a significantly adverse impact of monosomal karyotype as compared with other “unfavorable” karyotypes. Further data to address the prognostic and predictive significance of specific cytogenetic abnormalities in sAML will be obtained from a currently enrolling 450-patient phase III randomized trial of amonafide + cytarabine vs daunorubicin + cytarabine in patients with sAML (ACCEDE).
Downie:Antisoma: Research Funding. Erba:Lilly: Research Funding; Antisoma: Research Funding; Wyeth: Research Funding; Cephalon: Honoraria, Research Funding; MGI Pharma: Honoraria; Pharmion: Honoraria; Celgene: Honoraria; BMS: Honoraria; Novartis: Honoraria, Research Funding; Genzyme: Consultancy, Honoraria, Research Funding; Gemin-X: Research Funding; Kanisa: Research Funding. Stone:Celgene: Consultancy, Speakers Bureau; Merck: Consultancy; Genzyme: Consultancy; Eisai: Consultancy. Rizzieri:Antisoma: Research Funding. Foran:Antisoma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal