Abstract
Abstract 2045
Poster Board II-22
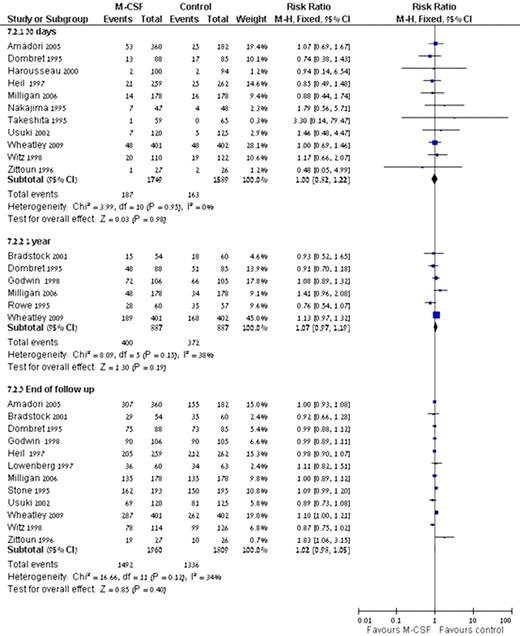
Myeloid colony-stimulating factors (M-CSFs) are recommended as primary prophylaxis for the prevention of febrile neutropenia (FN) in patients who are at high risk for developing it. Since acute myeloid leukemia (AML) cells express functional growth factor receptors on their surface, concerns have been raised regarding the effect of M-CSFs on these cells. We assessed the safety of M-CSFs in AML patients. Systematic review and meta-analysis of all randomized controlled trials that compared the addition of M-CSFs during and following chemotherapy to chemotherapy alone in patients with AML was conducted. Trials evaluating the role of M-CSFs administered for the purpose of stem cell collection and/or priming e.g., before and/ or only for the duration of chemotherapy, were excluded. Both patients with and without neutropenia and/ or fever on admission were included. Two reviewers appraised the quality of trials and extracted data independently. The primary outcome was all-cause mortality at defined points in time. Secondary outcomes included complete remission (CR), disease free survival (DFS), relapse and infection rates. Relative risks (RR) with 95% confidence intervals (CIs) were estimated and pooled. The search yielded 17 trials including 4800 patients (range 53-803 per trial). 14 trials included patients undergoing induction chemotherapy, two trials included patients undergoing consolidation and one trial included patients undergoing salvage chemotherapy. The addition of M-CSFs to chemotherapy yielded no difference in all-cause mortality at 30 days, 1 year and at the end of follow-up [RR 1.00 (95% CI.0.82-1.22), RR 1.07 (95% CI 0.97-1.19) and RR 1.02 (95% CI 0.98-1.05), respectively] (Fig.1). There was no difference with regard to CR [RR 1.03 (95% CI 0.98-1.07)], relapse [RR 0.99 (95% CI 0.91-1.08)] and DFS [HR 1.00 (95% CI 0.90-1.13)]. M-CSFs did not decrease the occurrence of bacteremias, RR 0.96 (95% CI 0.82-1.12) or invasive fungal infections, RR 1.40 (95% CI 0.90-2.19). The addition of M-CSFs to chemotherapy does not affect all-cause mortality and leukemia associated clinical end points, e.g. CR, relapse rate and DFS. Hence, the use of M-CSFs for prevention of FN in patients with AML is safe. However, since prevention of neutropenia does not result in improved survival, they should not be used on a routine basis. Their use in the individual patient can be considered according to the clinical situation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal