Abstract
Abstract 2032
Poster Board II-9
Hepatotoxicity is a potential complication of both methotrexate (MTX) and 6-mercaptopurine (6-MP) treatment. Historically, 6-MP is given on a once daily basis (75 mg/m2), but because 6-MP is S-phase dependent and has an extremely short half-life, POG 9065 tested twice daily 6-MP dosing (37.5 mg/m2) in order to achieve increased 6-MP cell perfusion/kill without augmentation of toxicity. POG 9605 was a phase III randomized 2×2 multifactorial study for standard risk childhood acute lymphoblastic leukemia (ALL) open from 1996-1999. The major objectives were to compare: 1) the same total dose 6-MP given on a once vs. twice a day schedule, and 2) the addition of 6 months of late intensification with biweekly divided dose oral methotrexate (ddMTX) (25 mg/m2 every 6hrs x 4 doses) to traditional weekly intramuscular MTX (IM MTX) (20mg/m2).
1077 charts from POG 9605 were reviewed to answer the following questions: 1) Do higher levels of plasma MTX during early intensification with intermediate dose methotrexate (IDMTX, 1gm/m2/24 hrs) predict increased later hepatotoxicity? 2) Are there differences in hepatotoxicity among the 6-MP and MTX regimens in late intensification? 3) 6-MP metabolite levels had been previously obtained for a subset of patients (226/1077). Since 6-MP metabolites were higher in once daily than twice daily dosing during continuation, would hepatotoxicity also be higher?
Hepatocyte damage (ALT, AST) and liver synthetic ability (albumin) were evaluated at 11 time points: beginning of consolidation (wk 5), during MTX infusions in consolidation (wks 7, 10, 13, 16, 19, 22), beginning and end of late intensification (wks 25, 49), and beginning and middle of continuation (wks 57, 85). MTX levels were recorded at the end of MTX infusions. Complete data points were obtained from 819/1077 charts. 26 children with Trisomy 21 received 50% doses of IDMTX during early consolidation. AST/ALT and albumin toxicities greater than or equal to grade 3 (NCI, CTC v2.0) were recorded. A mixed model repeated measures Analysis of Variance was used to analyze the data.
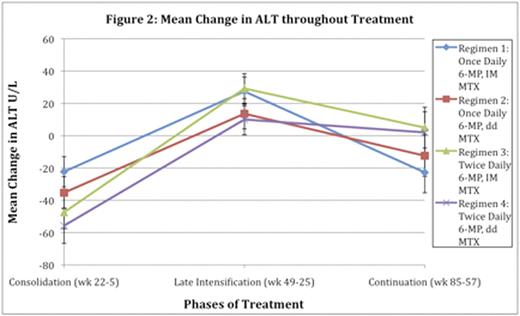
Mean AST and albumin levels were normal for all weeks during IDMTX infusions in consolidation. Mean end infusion MTX levels during this phase ranged from 10-12μM for patients without Trisomy 21 and 7-9μM for patients with Trisomy 21. Mean ALT was elevated but not above 100 U/L and no correlation was found between mean MTX levels and mean ALT, AST, or albumin levels. In late intensification and continuation, no significant differences in mean ALT, AST, and albumin levels were found between once daily and twice daily 6-MP dosing. There was no correlation between 6-MP metabolite levels and liver enzyme levels during continuation. For the MTX regimens, there was no significant difference in mean liver enzyme levels between weekly IM MTX and biweekly oral ddMTX during late intensification (Figure 1 & 2). For the time points analyzed, of the 793 patients without Trisomy 21, 4.0% had AST levels greater than or equal to 5X the normal upper limit. 18.3% had ALT levels greater than or equal to 5x and less than 10x the normal upper limit and 3.2% had ALT levels greater than or equal to 10x the normal upper limit. Despite these numbers, there were no significant differences in the percent of patients with elevated ALT and AST levels among the different dosing arms. Mean ALT and AST levels for all patients did not go above 120 U/L and 57 U/L, respectively.
No significant difference was found in the number of AST/ALT and albumin recorded toxicities among the different 6-MP and MTX regimens. Of the 793 patients without Trisomy 21, the lowest number of toxic events occurred during consolidation at 244 events for AST/ALT and 3 events for albumin. Continuation had the highest number of toxic events, at 388 events for AST/ALT and 6 events for albumin, possibly due to the accumulation of toxicity from previous phases of therapy and the longer length of continuation. The mean number of toxic events experienced for all patients was less than 1 event for each phase of treatment. There was no significant difference between the number of AST/ALT and albumin toxic events experienced between Trisomy 21 and patients without Trisomy 21.
Divided dose 6-MP and MTX as used on POG 9605 were not more hepatotoxic when compared to traditional 6-MP and MTX dosing. These new dosing regimens, if found to be more efficacious, could be applied to future ALL treatments.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal