Abstract
Abstract 2017
Poster Board I-1039
Hypogonadotropic hypogonadism (HH) is the most common endocrine problem in chronically transfused patients. Unfortunately, gonadotrophe iron toxicity is not readily detectable until puberty and stimulation tests can be difficult to interpret in adolescents. MRI can image preclinical pituitary iron deposition, similar to its use in the heart, liver and pancreas. Increased pituitary R2, a surrogate for iron, and decreased pituitary volume have both been shown to predict clinical and biochemical HH in iron overloaded adults 1,2. However, data regarding pituitary iron deposition and toxicity is lacking in younger patients with iron overload. We present baseline results from a two year observational trial of changes in pituitary R2 and volume in response to deferasirox therapy in patients with transfusional siderosis.
We studied 22 chronically-transfused patients with Thalassemia Major and 2 patients with Diamond Blackfan Anemia. The average age was 13.1 ± 5.2 years (range: 3.7-23.6 years). All studies were performed on a 1.5 T Philips Achieva. Anterior pituitary R2 was assessed in the sagittal and coronal planes using multiple spin echoes from 15 to 120 ms. Pituitary volume was assessed using a 3D spoiled gradient echo sequence with 1 mm3 isotropic voxels. Pituitary R2 was calculated by pixelwise monoexponential fit, with median values used to represent the overall gland R2; boundaries were confirmed by a board-certified neuroradiologist. Normative data for pituitary iron and volume was drawn from another study in 49 normal volunteers. MRI estimates of hepatic iron concentration (HIC), cardiac iron (T2*), and pancreatic iron (R2*) were obtained as clinical standard of care. All statistics were performed using JMP5.1 (SAS, Cary, NC).
Patients were mild to moderately iron loaded, with a HIC of 10.2 ± 12.0 mg/g dry wt (median 4.5 mg/g, nl < 1.5 mg/g), cardiac T2* of 29.4 ± 9.2 ms (median 31.5 ms, nl > 20ms), and pancreas R2* of 136 ± 156 Hz (median 73 Hz, nl < 27 Hz). Fourteen patients were below the 10th percentile for height including 7 below the 5th percentile. One patient had been diagnosed with delayed puberty and was on estrogen replacement.
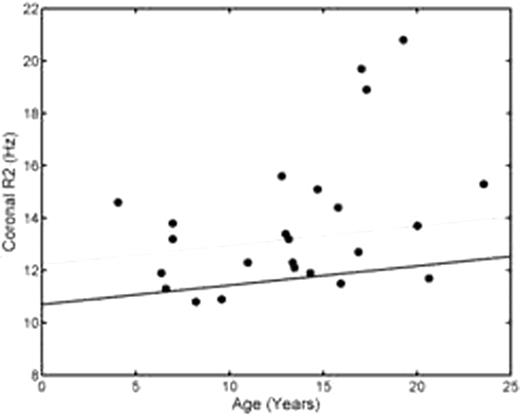
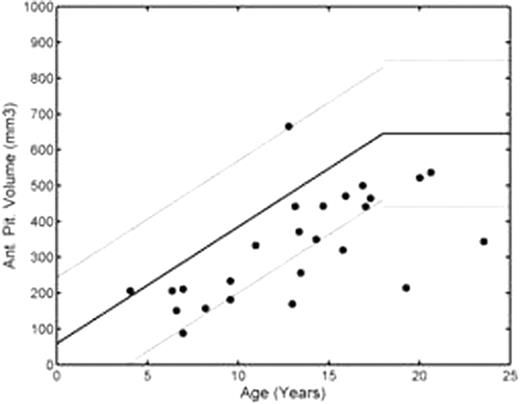
Pituitary R2 was elevated in 13/24 patients, beginning in the first decade of life and worsening in patients older than 13 years of age (Figure 1, left). Mean Z-score was 3.2 ± 3.7 (median 2.5, range -0.5 to12.8). Pituitary R2 was correlated with HIC (r2=0.68) and pancreatic R2* (r2=0.40), but not cardiac R2*. Area under the receiver operator characteristic curve was 0.76 for both HIC and pancreatic iron for the prediction of pituitary iron loading. Anterior pituitary volumes were low-normal in the first decade of life but were below the 1st percentile in 4/16 patients older than 13 years of age (Figure 1, right); all 4 patients with severe pituitary shrinkage had significant pituitary iron loading (Z-scores ranging from 2.8 to 12.8). The patient having documented HH had an iron Z-score of 12.8 and a volume Z-score of –5.0.
HH remains one of the most difficult endocrinopathies to recognize and prevent in transfusional siderosis. The present data demonstrate that iron accumulation occurs early in these patients and worsens dramatically in the second decade of life. Pituitary R2 was closely correlated with liver and pancreatic iron suggesting more rapid iron transport kinetics than for the heart. The low-normal pituitary volumes in childhood may reflect ethnic mismatches between the control and study populations, early iron toxicity, or hypothalamic dysfunction from increased erythropoiesis and metabolic demand. However, severe pituitary volume loss was limited to the second decade of life and associated with markedly increased pituitary iron (R2). Thus, there appears to be a broad, preclinical window to reduce pituitary toxicity. MRI screening of pituitary size and iron concentration needs to be expanded, clinically, to explore this hypothesis and hopefully prevent the significant physiological and psychological sequelae associated with hypogonadism.
Coates:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Wood:Novartis: Research Funding.
References
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal