Abstract
Abstract 1931
Poster Board I-954
Indoleamine 2,3-dioxygenase (IDO),the key enzyme in tryptophan metabolism, catalyzes the initial rate-limiting step of tryptophan degradation along the kynurenine pathway. Tryptophan starvation by IDO consumption and products of tryptophan catabolism can inhibit T-cell activation, regulate T-cell proliferation and survival, and participate in tumor-induced tolerance. This effect can be abrogated by IDO inhibitor, 1-methyl tryptophan (1-MT), which has been proved to possess an anti-tumor effect. In this study, we aim to measure the expressions of IDO and Foxp3 in NHL and to investigate in-vitro whether T-cell tolerance in NHL may be induced by directly converting CD4+CD25- T cells into CD4+CD25+ Treg cells through an IDO-dependent mechanism.
Twenty fresh and fifty-seven paraffin embedded NHL tissues were collected. Reactive lymphadenitis tissues were set as control group. Quantitative expressions of IDO and Foxp3 mRNA in NHL tissues and counterparts were assessed by real-time PCR. IDO and Foxp3 protein expressions were investigated by both immunohistochemistry staining and Western-blot. Murine CD4+CD25- T-cell isolated by MACS and identified by FACS were co-cultured with IDO-expressing A20 cell line in the presence and absence of 1-MT, which specially inhibits IDO activity.
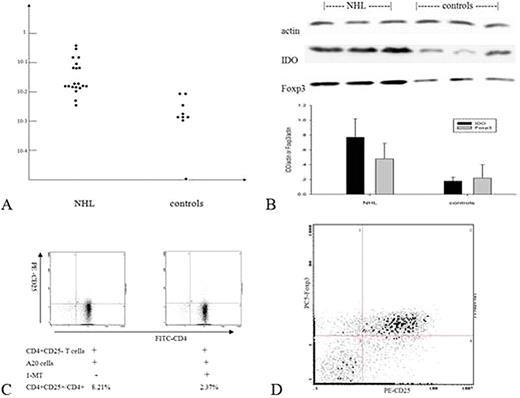
IDO mRNA was up-regulated expression in NHL tissues compared to non-neoplastic counterparts(2-dCt: 0.00582∼0.546 v. 0∼0.0103, p<0.01, Fig 1A). A significant correlation was found between higher IDO mRNA level and Foxp3 mRNA expression. In IHC (Table.1), cytoplasmatic IDO staining was positive in 50 NHL specimens, in whch eighteen were strong-stained. The proportion of Foxp3+ cells was also higher in NHL patients than controls (Ö2=5.28,P<0.05). Interestingly, there were more Foxp3+ cells in IDO strong staining tissues than week staining. IDO protein level detected by WB was coincidentally higher in NHL than controls(IDO/b-actin: 0.77±0.25 v. 0.18±0.18, p<0.01) and with elevated IDO protein level, Foxp3 protein expression was markedly increased(Fig.1B). Co-culture of CD4+CD25- T cells with A20 cells increased the surface expression of CD25 and the percentage of CD4+CD25+ T cells (8.21%). The addition of 1-MT attenuated conversion of CD4+CD25- to CD4+CD25+ T cells (2.37%), whereas 1-MT had no solitary effect on CD4+CD25- T cells(Fig. 1C). In addition, it was confirmed by FACS that most of the converted CD4+CD25+ T cells expressed Foxp3, which was the representative transcriptive factor of Tregs(Fig. 1D).
| Group . | IDO . | P value . | |
|---|---|---|---|
| Strong staining (positive cell rate≥30%) . | Week/Negative staining (positive cell rate<30%) . | ||
| Control group | 0 | 18 | |
| NHL | 18 | 39 | P<0.05a |
| Foxp3-strong staining (positive cell rate≥3%) | 16 | 10 | |
| Foxp3- week/negative staining (positive cell rate<30%) | 2 | 29 | P<0.05b |
| Group . | IDO . | P value . | |
|---|---|---|---|
| Strong staining (positive cell rate≥30%) . | Week/Negative staining (positive cell rate<30%) . | ||
| Control group | 0 | 18 | |
| NHL | 18 | 39 | P<0.05a |
| Foxp3-strong staining (positive cell rate≥3%) | 16 | 10 | |
| Foxp3- week/negative staining (positive cell rate<30%) | 2 | 29 | P<0.05b |
Chi-square analysis,
Fisher's exact probabilities in 2°Á2 table
Both mRNA and protein of IDO are increasingly expressed in NHL, synchronously with the over-expression of Foxp3. Up-regulation of IDO may induce T-cell tolerance by directly increasing Treg cells through the conversion of CD25- into CD25+ T cells. This could be regarded as a novel mechanism of NHL escape from immune control and its inhibition may represent a novel anti-tumor therapeutic strategy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal