Abstract
Abstract 1864
Poster Board I-889
In preclinical models lenalidomide has been shown to augment immune responses in vitro while the in vivo immunomodulatory properties of this drug are unknown. This clinical trial in relapsed myeloma patients examined the ability of lenalidomide to augment both endogenous as well as vaccine-specific cellular and humoral immune responses to the pneumococcal vaccine, Prevnar.
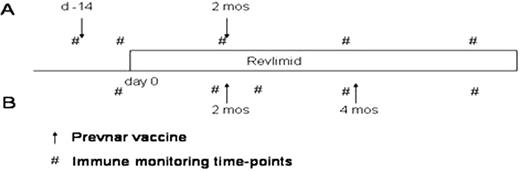
Prevnar was given either before or during administration of lenalidomide in two cohorts of patients. Cohort A received their first vaccination prior to administration of lenalidomide, and the second vaccine on cycle 2, day 15 of lenalidomide. Cohort B received both vaccines after initiation of lenalidomide: cycle 2, day 15 and cycle 4, day 15. Pneumococcal serotype titres as well as CRM-197 T cell responses quantified the B and T cell responses, respectively, to Prevnar vaccination and were correlated with lenalidomide administration. Systemic immune responsiveness was determined by DTH responses to candida and by flow cytometric analysis of immune cell subsets.
Enrollment has been completed. 24 patients have been enrolled. 17 patients were evaluable with 10 in Cohort A and 7 in Cohort B. 7 patients with progressive disease while on study were not evaluable. All patients had measurable T cell and antibody responses to Prevnar. T cell responses to CRM-197 demonstrated significantly higher responses in Cohort B with a peak 5.8 fold-increase above baseline compared to 1.5 in Cohort A in the blood. The bone marrow T cells showed a greater response which persisted longer in Cohort B (15.5 fold to 4.5 at 6 months) vs. A (12.5 fold to 1.8 at 6 months). Trends towards better T cell responses were observed in patients with better clinical responses to lenalidomide. Vaccination primed serotype responses in both groups. While the second Prevnar vaccine in Cohort B further augmented antibody titres, no subsequent increase was observed in Cohort A. As a measure of systemic immunity, DTH responses to Candida were examined. Cohort B demonstrated up to a 78-fold increase in induration compared to no change in Cohort A. Flow cytometric analyses showed increased NK (1.2 fold) and CD8 (2.5 fold) in Cohort B… Furthermore, T cell subset analyses revealed an increase of activation markers as well as an increase in the central memory and effector memory phenotypes in both peripheral blood and bone marrow. Interestingly, greater myeloma clinical responses were observed in Cohort B (57% ORR) vs Cohort A (10%)
This is the first in vivo demonstration of lenalidomide-mediated augmentation of both cellular and humoral responses in myeloma patients. These data suggest a synergy between the immunomodulatory effects of lenalidomide and vaccines. Surprisingly, the increased anti-tumor effect observed in Cohort B suggests the possibility of lenalidomide-induced vaccine-mediated epitope spreading. This establishes the scientific rationale for utilizing lenalidomide as an immune adjuvant with vaccines. Implications of the use of lenalidomide as a vaccine adjuvant apply to both cancer as well as infectious vaccines.
Off Label Use: Lenalidomide as an immune potentiator. Huff:Celgene: Consultancy. Schafer:Celgene: Employment. Borrello:Celgene: Speakers Bureau; Mellenium: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal