Abstract
Abstract 1768
Poster Board I-794
Assessment of the safety profile, in particular renal function, of the oral iron chelator deferasirox (Exjade®) is of particular relevance in MDS patients (pts), given their advanced age and associated decline in renal function. This 1-yr pooled analysis characterizes the safety profile of deferasirox in a large population of MDS and non-MDS pts (β-thalassemia [β-thal] and other anemias [sickle cell, Diamond-Blackfan, aplastic and other]), with emphasis on renal function.
Analysis is based on 1-yr pooled data from iron-overloaded pts who were enrolled in five open-label deferasirox studies: US02 and US03 (single-arm (SA), Low/Int 1 MDS pts, starting dose 20 mg/kg/day); 2409 (SA, pts with transfusional hemosiderosis, starting dose 20 mg/kg/day); 107 (randomized trial in β-thal pts, dosing 5–40 mg/kg/day) and 108 (SA, pts with chronic anemias, dosing 5–40 mg/kg/day). Datasets pooled: baseline (BL) characteristics, dosing, transfusion, adverse events (AEs) and laboratory data.
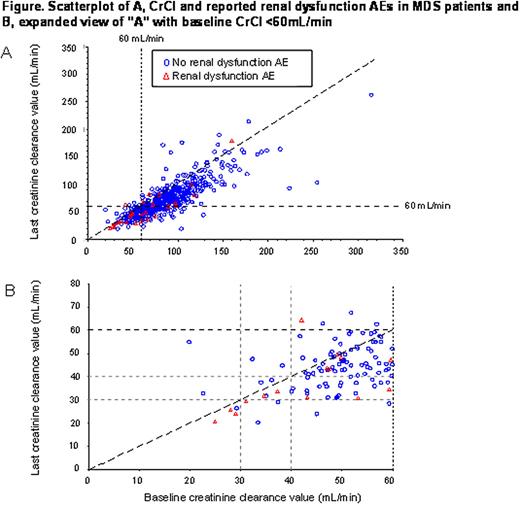
1798 pts were assessed (951 β-thal; 584 MDS, 263 other). MDS pts were older (median age 71 yrs) compared with those with β-thal (25 yrs) and other anemias (38 yrs). Mean actual dose of deferasirox (mg/kg/day): β-thal pts 23.5; MDS pts 20.3; other 19.9. Mean transfusional iron intake (mg/kg/day): 0.34, 0.32 and 0.23 in β-thal, MDS and other anemias, respectively. At 1 yr, 88%, 53% and 69% of β-thal, MDS and other pts remained in the study, respectively. Most frequent reasons for discontinuation: AEs (n=42 [4%] β-thal; n=115 [20%] MDS; n=34 [13%] other anemias). Frequency of drug-related AEs was similar for MDS (68%) and other anemias (63%) compared with 49% for β-thal. Most common drug-related AEs in β-thal, MDS and others, respectively, were: diarrhea (11%; 37%; 23%), nausea (7%; 15%; 20%), vomiting (3%; 8%; 8%); rash (11%; 8%; 6%); abdominal pain (6%; 7%; 8%) and upper abdominal pain (4%; 5%; 8%). 64 deaths occurred (none assessed as related to deferasirox by the investigator); 4 (primarily cardiac), 47 (primarily infections, hemorrhages, progression to AML, cardiac events) and 13 (primarily infections) in the β-thal, MDS and other pts, respectively. BL versus last available creatinine clearance (CrCl) were analyzed graphically for MDS pts with/without investigator-reported renal dysfunction AEs (Figure). CrCl for the majority of β-thal and non-MDS pts was >60 mL/min at BL (not shown), while in the MDS group, as expected, there were more pts with CrCl <60 mL/min and reported renal dysfunction AEs (Fig. A). To evaluate pts with BL CrCl <60 mL/min, an expanded view was created (Fig. B). A greater proportion of MDS pts with renal AEs was reported in the 20–<30 mL/min (43%) and 30–<40 mL/min category (30%), versus the 40–<50 mL/min (13%) and 50–<60 mL/min categories (7%). Exploratory logistic regression modeling confirmed odds of reporting a renal dysfunction AE were 4 and 22 times for pts with CrCl 40–<60 mL/min and <40 mL/min, respectively, compared to pts with CrCl ≥60 mL/min. Pts with BL CrCl <40 mL/min (n=6) were >70 yrs with primarily pre-existing chronic renal insufficiency, diabetes, hypertension and congestive heart failure. Pts with BL CrCl 40–60 mL/min had fewer pre-existing conditions, but still had reported renal dysfunction AEs. In about half of cases with BL CrCl <60 mL/min, reported renal dysfunction AEs were not associated with a clinically significant increase in SCr and corresponding CrCl decline, consistent with investigators' discretion in reporting.
This comprehensive assessment of MDS and non-MDS pts with transfusional iron overload confirmed the known safety profile of deferasirox; with the most frequent drug-related AEs reported as gastrointestinal events. The findings were also consistent with the clinical features of MDS such as advanced age and co-morbidities. Pts with BL CrCl <40 mL/min were more likely to report renal dysfunction AEs and less likely to be able to compensate for additional stresses on their existing decreased renal function, given their pre-existing co-morbidities and advanced age. Deferasirox may be used in pts with BL CrCl 40–<60 mL/min with close monitoring, and should not be used in pts with BL CrCl <40 mL/min.
Cappellini:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genzyme: Membership on an entity's Board of Directors or advisory committees. Porter:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Vifor International: Membership on an entity's Board of Directors or advisory committees. Greenberg:Amgen: Consultancy, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Lawniczek:Novartis Pharma AG: Employment. Glaser:Novartis Pharma AG: Employment. Dong:Novartis Pharmaceuticals: Employment. Gattermann:Novartis: Honoraria, Participation in Advisory Boards on deferasirox clinical trials.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal