Abstract
Abstract 1720
Poster Board I-746
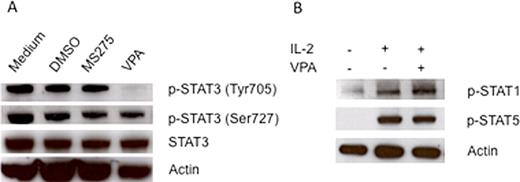
Valproic acid (VPA), a histone deacetylase (HDAC) inhibitor (1), was found to enhance NK cell mediated antitumor activity by upregulating the surface expression of NKG2D ligands MICA and MICB on hepatoma cells (2). In contrast, VPA was found to impair NK cell antitumor activity by downregulating the surface expression of NKp30 and NKp46 on NK cells by blocking NFkB activation (3). Recently, we also found that VPA efficiently upregulates the surface expression of MICA, MICB, and ULBP family ligands on human osteosarcoma and neuroblastoma cells, but downregulates NKG2D surface expression on human NK cells. Similarly, the HDAC inhibitor MS275 efficiently upregulates expression of MICA, MICB, and ULBP family ligands on human osteosarcoma and neuroblastoma cells, but surprisingly, MS275 significantly upregulates NKG2D expression on NK cells. In addition, we found that VPA inhibited the cell growth of osteosarcoma cell lines more readily than MS275. To investigate the mechanism of these differing effects of VPA and MS275, we compared the effect of these HDAC inhibitors on Signal Transducer and Activator of Transcription (STAT) signaling, which are important signaling pathways in NK cells and many cancers. Here we show that VPA is a potent inhibitor of STAT3 phosphorylation at the tyrosine 705 residue in NK cells, whereas MS275 has no effect on STAT3 phosphorylation (Figure A). Moreover, this inhibition is highly specific, as VPA has no effect on STAT1 and STAT5 phosphorylation (Figure B). To further confirm VPA-mediated STAT3 inhibition, we detected STAT3 expression and the degree of tyrosine phosphorylation affected by VPA in a panel human osteosarcoma cells. We found that constitutive STAT3 phosphorylation is present in osteosarcoma SaOS2 and LM7 cells, but not in OS187 cells, which corresponded to the stronger growth inhibition caused by VPA in SaOS2 and LM7 cells compared to OS187 cells. These results are for the first to show that VPA, a HDAC inhibitor, is also a selective STAT3 inhibitor. VPA can upregulate NKG2D ligand expression on the tumor cell surface by HDAC inhibition, but may downregulate NKG2D expression on the NK cell surface by STAT3 inactivation. The antitumor activity of VPA may be via a mechanism of both HDAC inhibition and STAT3 inactivation. The knowledge of potent STAT3 inhibition by VPA is important for identifying potential new clinical applications for this drug.
No relevant conflicts of interest to declare.
References
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal