Abstract
Abstract 170
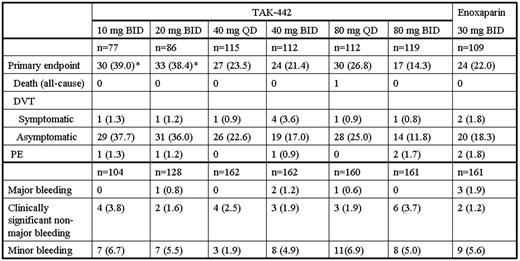
TAK-442 is an oral, selective, direct competitive inhibitor of activated factor X (fXa). This phase 2, multicenter, randomized, parallel-group study compared a range of TAK-442 doses with enoxaparin for thromboprophylaxis after elective unilateral total knee arthroplasty. After surgery, males and females 18 years of age or older were randomly assigned to 1 of 7 treatment groups: TAK-442 at doses of 40 or 80 mg p.o. once daily (QD) or 10, 20, 40, or 80 mg p.o. twice daily (BID), or enoxaparin 30 mg s.c. BID (n=150 per group). Enoxaparin was given unblinded, whereas TAK-442 given double-blinded with regard to dose and regimen. The first dose of TAK-442 was administered 6-8 hours postoperatively and after hemostasis was established, while the first dose of enoxaparin was given 12-24 hours after surgery according to North American guidelines; TAK-442 and enoxaparin were given for 10 days and patients were followed for 30 days after the last dose of study drug. Bilateral ascending venography was performed after the last dose of study drug to screen for deep vein thrombosis (DVT). The primary efficacy endpoint was the composite of all-cause mortality, symptomatic and asymptomatic DVT, and pulmonary embolism (PE). The primary safety endpoint was major bleeding, based on a pre-specified bleeding scale. Independent, blinded committees adjudicated all suspected efficacy outcomes and bleeding events. Of the 1038 patients who received study drug and were included in the full analysis set (FAS) and safety population, 949 completed the study; 730 patients had evaluable venograms. Following the recommendation of the Data Safety Monitoring Board, the TAK-442 10 mg and 20 mg BID groups were discontinued prematurely because of lack of efficacy with respect to asymptomatic DVT. The incidences of the primary efficacy endpoint and its components in the population with evaluable endpoints, and of major bleeding in the safety population, are shown in the table.
Patients in the FAS exhibited a similar trend in efficacy, with incidences of the composite primary endpoint of 28.8%, 25.8%, 16.7%, 14.8%, 18.8%, and 10.6%, respectively, across the TAK-442 doses, and 14.9% for enoxaparin. The minimum effective dose of TAK-442 was determined to be 40 mg QD, while the maximum dose of 80 mg BID tended to be more effective than enoxaparin in this study. The frequency of major and clinically significant non-major (CSNM) bleeding was not dose-dependent or different from that seen with enoxaparin. Similarly, the frequency of liver function test abnormalities was not dose-dependent or different from that seen with enoxaparin. Treatment-emergent AEs leading to study drug discontinuation occurred in 5.5% of the TAK-442 subjects and 7.5% of the enoxaparin subjects. In addition, the overall incidence of AEs or SAEs in the TAK-442 treatment groups was not dose-dependent or greatly different from that seen with enoxaparin 30 mg BID. These data suggest that, at total daily doses of 40 to 160 mg, the efficacy of TAK-442 is similar to that of enoxaparin 30 mg BID in patients undergoing total knee replacement. The low incidence of major and CSNM bleeding suggests that TAK-442 has a favorable benefit to risk profile in this population.
Weitz:Daiichi-Sankyo: Consultancy; Bayer: Consultancy; BMS: Consultancy; Pfizer: Consultancy; Boehringer-Ingelheim: Consultancy; Takeda Global Research & Development, Inc.: Consultancy; Astra-Zeneca: Consultancy; The Medicines Company: Consultancy; Merck: Consultancy. Cao:Takeda Global Research & Development, Inc.: Employment. Eriksson:Takeda Global Research & Development, Inc.: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Boehringer Ingelheim: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Fisher:Bristol Myers Squibb: Research Funding; Takeda Global Research & Development, Inc.: Honoraria; Bayer: Consultancy, Honoraria, Research Funding; sanofi-aventis: Honoraria, Research Funding, Speakers Bureau; Boeringer-Ingelhiem: Consultancy, Speakers Bureau. Kupfer:Takeda Global Research & Development, Inc.: Employment. Raskob:Takeda Global Research & Development, Inc.: Consultancy; Bayer: Consultancy; BMS: Consultancy; Boehringer –Ingelheim: Consultancy; Daiichi-Sankyo: Consultancy; Johnson and Johnson: Consultancy; Pfizer: Consultancy; sanofi-aventis: Consultancy. Spaeder:Takeda Global Research & Development, Inc.: Employment, Equity Ownership. Turpie:Takeda Global Research & Development, Inc.: Consultancy; Astellas: Consultancy; Portola: Consultancy; Bayer: Consultancy; Johnson & Johnson: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal