Abstract
Abstract 1639
Poster Board I-665
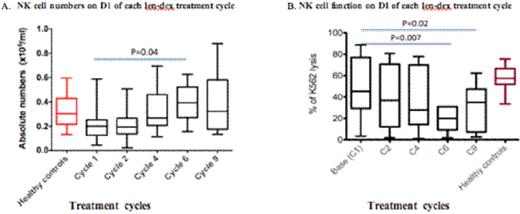
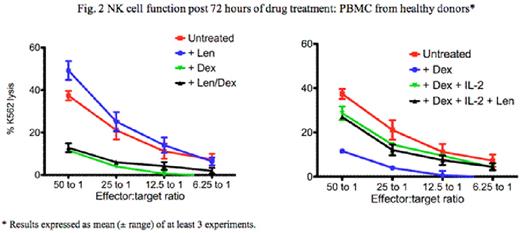
Dexamethasone (dex) and lenalidomide (len) is a potent treatment for multiple myeloma (MM). In vitro, len directly inhibits MM tumor cell proliferation via cell cyle arrest, and can also costimulate T cells and augment natural killer (NK) cell activity, leading to enhanced anti-tumour immunity. Conversely, dex also directly inhibits MM cell proliferation but is profoundly immuno-suppressive and may therefore subvert the full capacity of len to act via immune mechanisms against MM. We previously reported that MM patients responding to len-dex combination show an increase in Treg numbers, and little evidence in recovery of their B and T cell numbers (Quach et al. Blood 2008; 112: abstract 1696). We have since undertaken a prospective and systematic analysis of NK cell number and function in MM patients treated with len-dex, and evaluated the mechanisms by which dex downregulates len-induced NK activation in in vitro assays using patients' and normal donors' blood samples. 25 relapsed MM patients (aged 58-77 years) were treated with low dose len (15mg Days 1-21 of each 28-day cycle) and dex (20mg/day, Days 1-4,9-12,17-20). After a median of 9 (2-19) cycles, 19 patients responded (24% CR/VGPR, 52% PR). At baseline, NK cell numbers and function [assessed by % lysis of 51Cr labelled K562 target cells at 50 (effector):1 (target) ratio] in MM patients were similar to age matched controls (0.2 vs. 0.3× 105/ml in controls, p=0.09 and 49% K562 cell lysis vs. 58% in controls, p=0.44 respectively) (fig.1A). Whilst NK cell numbers slightly increased in vivo after len-dex treatment [2.0 (baseline) vs. 3.9×105/l (cycle 6), p=0.04, paired t test] (fig.1A), mean NK cell function progressively decreased compared to baseline after 6 and 9 len-dex cycles [mean 49% K562 cell lysis at baseline vs. 28% after 6 cycles (p=0.007) and 31% after 9 cycles (p=0.02)] (fig.1B). Following 72 hours of in vitro treatment with len (10mM), there was increased NK function in healthy donor peripheral blood mononuclear cells (PBMC) [mean 54% K562 cell lysis from len-treated PBMC vs. 38% lysis in untreated PBMC, p=0.04] (fig. 2). In PBMCs from MM patients at baseline, ex vivo treatment with len (10mM) did not significantly increase NK cell function [mean 47% K562 cell lysis (untreated) vs. 52% (len treated), p=0.17], nor did it increase NK cell function after 6 len-dex treatment cycles [mean 32% K562 cell lysis (untreated) vs. 30% (treated), p=0.4].Conversely, dex (0.1mM) decreased NK cell function in healthy donors' PBMC [mean 7.6% K562 cell lysis (dex treated) vs. 38% (untreated) p=0.01], even in the presence of len [mean 7% K562 cell lysis (len+dex) vs. 38% (untreated), p=0.002] (fig. 2). Dex-induced in vitro NK inhibition was dose dependent and could be rescued by the addition of IL-2 to normal donors [mean 7.6 % K562 cell lysis (dex) vs. 28% lysis (Dex +IL2),p=0.03] as well as PBMC from MM patients at baseline [mean lysis 16% (dex) vs. 59% (Dex+IL2) p=0.0002]. However, IL-2 was less able to rescue dex-induced NK dysfunction in PBMC from patients post 6 treatment cycles compared to patients at baseline [mean 59% K562 cell lysis (baseline) vs. 28% (C6), p=0.03]. Dex induced NK dysfunction was reversible as NK cell function recovered after a 3 days dex washout. In summary, NK function in MM patients, whilst similar to healthy controls at baseline, progressively decreases after prolonged len-dex treatment despite a clinical response. The observed decrease in NK function in vivo and in vitro is directly due to the effects of dex, which could not be reversed by the NK activating effects of len. Our results suggest that the efficacy of len and dex co-therapy is not due to augmentation of NK cytolytic activity, due to the immunosuppressive effects of dex against NK cells. This suggests that alternative dosing schedules of dex, after initial induction with len and dex co-therapy, may optimise len-induced immunostimulation of NK cells and subsequent sustained disease control via anti-MM immunity.
Lynch:Celgene Corporation: Employment. Prince:Celgene Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal