Abstract
Abstract 1418
Poster Board I-441
Seventy percent of clinical trials in the United States are delayed by one to six months due to enrollment challenges. Barriers to accrual are site specific as well as protocol specific. Site specific challenges include perceptions of patient ineligibility and cumbersome screening processes. Protocol barriers include complexity of inclusion and exclusion criteria. Coomis et al (J Onc Prac 2009; 5:50) reported that 73% of overall clinical trial awareness was generated by physician interest. Therefore, the familiarity of investigators with each trial is crucial to optimizing patient accrual. The standard protocol initiation process relies on investigators and staff to attend an investigator's meeting. However, this approach does not focus on actual clinical patient presentations to determine trial eligibility or exclusion. As a result, many investigators may overlook key criteria for eligibility thus losing patients to enrollment. We have previously shown that interventions directly with PIs to increase PI awareness of inclusion/exclusion criteria of a clinical trial results in improved trial accrual (ASCO 2009, abs 6613).
Investigator focused Accrual Workshops (AW) are used to increase patient enrollment to oncology clinical trials. While a standard investigator meeting includes protocol review, AW first create a compelling scientific story to re-engage the investigators in the trial. The key to these AW is the use of case-based learning to highlight the clinical and scientific rationale of the trial as opposed to general protocol review.
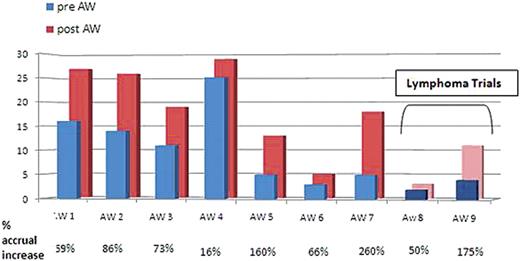
Between May 2008 and May 2009, 14 AW were conducted for 5 separate studies including 2 lymphoma trials. The AW with less than 120 days of follow up (n=5) show no significant impact although there was a trend toward increased accrual. In those with more than 120 days of follow up (n=9), the mean increase in accrual was 108% with increased accrual following every AW (Figure 1). After those 9 AW, patient accrual per month increased 1.65 fold on average. Analysis of the 2 lymphoma clinical trials demonstrates a significant increase in accrual following AW of 230% and 40%, respectively.
AW using physician to physician clinical case-based learning provide an effective means of educating investigators and research staff in the identification of patients who meet the criteria for participation in clinical trials. The impact of AW on clinical trial accrual is best seen over time and they are effective in lymphoma clinical trials as well as others. After every AW with more than 120 day follow up, accrual was accelerated.
Accrual before and after 9 AW with a more than 120 day follow up shows a mean accrual increase of 108%.
Accrual before and after 9 AW with a more than 120 day follow up shows a mean accrual increase of 108%.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal