Abstract
Abstract 136
The serum free light chain (FLC) assay quantitates free kappa and free lambda immunoglobulin light chains. This assay has prognostic value in MGUS, multiple myeloma (MM), solitary plasmacytoma, and AL amyloidoisis, and has been incorporated in response criteria for both MM and AL amyloidosis. A percentage of non-Hodgkin's lymphoma (NHL) patients also present with abnormal FLC concentrations and recent data suggest that AIDS patients with elevated serum FLC are at higher risk of developing NHL. Here we assess the impact of FLC in a cohort of patients with diffuse large B-cell lymphoma (DLBCL) enrolled on a cooperative group clinical trial.
Newly diagnosed DLBCL patients were treated with epratuzumab + R-CHOP (ER-CHOP) on NCCTG clinical trial N0489. Serum FLC was quantitated prior to treatment and after cycles 2 and 6 using the Freelite FLC assay (The Binding Site, Ltd., Birmingham, UK). Patients were followed per clinical trial protocol for event-free (progression, retreatment, or death due to any cause) and overall survival (EFS and OS, respectively).
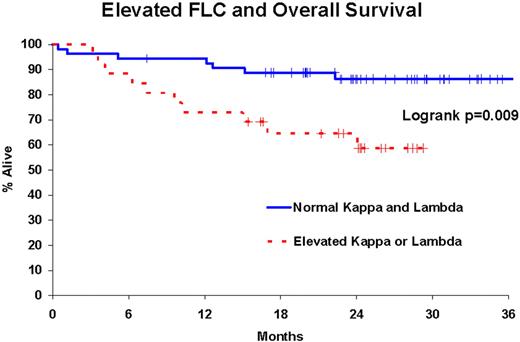
107 patients were enrolled on the trial. 80 patients had a pre-treatment serum draw and a central pathology confirmed diagnosis of DLBCL, of which 47 had a post-cycle 2 sample (C2) and a 54 had a post-cycle 6 sample (C6). At a medium follow-up of 25 months (range 7-39), 22 patients (28%) had an event and 17 patients died (21%). Prior to treatment 23 patients (29%) had abnormal kappa concentrations (all elevated), 16 (20%) had abnormal lambda concentrations (11 elevated, 5 decreased), and 9 (11%) had an abnormal kappa/lambda ratio. FLC concentrations were significantly reduced in patients during treatment, with a median reduction in kappa (C2: 46%, C6: 64%) and lambda (C2: 44%, C6: 49%), all p<0.0001. Elevated FLC prior to treatment was highly associated with both OS (logrank p=0.009) and EFS (logrank p=0.006); these associations remained significant after adjusting for IPI (both p = 0.01).
A significant percentage of DLBCL patients present with abnormal FLC concentrations. Kappa and lambda are significantly reduced for most patients during treatment. Elevated pre-treatment FLC concentrations are associated with inferior EFS and OS in DLBCL and this association is independent of the IPI. A follow-up study of FLC and prognosis is currently underway in a cohort of 1500 NHL patients from the Mayo Clinic and University of Iowa SPORE.
| Association of Pre-treatment FLC with Outcome . | N (%) . | OS HR (95% CI) . | EFS HR (95% CI) . |
|---|---|---|---|
| Elevated Kappa | |||
| Univariate | 23 (29%) | 3.3 (1.3, 8.5) | 2.5 (1.1, 5.9) |
| IPI Adjusted | 3.1 (1.2, 8.1) | 2.3 (1.0, 5.5) | |
| Elevated Lambda | |||
| Univariate | 11 (14%) | 3.1 (1.1, 8.8) | 4.2 (1.7, 10.4) |
| IPI Adjusted | 4.4 (1.5, 13.2) | 5.3 (2.1, 13.5) | |
| Abnormal Kappa/Lambda Ratio | |||
| Univariate | 9 (11%) | 2.8 (0.91, 8.6) | 1.9 (0.65, 5.7) |
| IPI Adjusted | 4.3 (1.4, 14.0) | 2.5 (0.82, 7.8) | |
| Elevated FLC (either) | |||
| Univariate | 26 (33%) | 3.4 (1.3, 8.9) | 3.1 (1.3, 7.2) |
| IPI Adjusted | 3.3 (1.3, 8.8) | 2.9 (1.3, 6.8) |
| Association of Pre-treatment FLC with Outcome . | N (%) . | OS HR (95% CI) . | EFS HR (95% CI) . |
|---|---|---|---|
| Elevated Kappa | |||
| Univariate | 23 (29%) | 3.3 (1.3, 8.5) | 2.5 (1.1, 5.9) |
| IPI Adjusted | 3.1 (1.2, 8.1) | 2.3 (1.0, 5.5) | |
| Elevated Lambda | |||
| Univariate | 11 (14%) | 3.1 (1.1, 8.8) | 4.2 (1.7, 10.4) |
| IPI Adjusted | 4.4 (1.5, 13.2) | 5.3 (2.1, 13.5) | |
| Abnormal Kappa/Lambda Ratio | |||
| Univariate | 9 (11%) | 2.8 (0.91, 8.6) | 1.9 (0.65, 5.7) |
| IPI Adjusted | 4.3 (1.4, 14.0) | 2.5 (0.82, 7.8) | |
| Elevated FLC (either) | |||
| Univariate | 26 (33%) | 3.4 (1.3, 8.9) | 3.1 (1.3, 7.2) |
| IPI Adjusted | 3.3 (1.3, 8.8) | 2.9 (1.3, 6.8) |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal