Abstract
Poster Board I-351
The detection of minimal residual disease (MRD) in AML patients after allogeneic stem cell transplantation (SCT) is important for the early detection of relapse and the selection of patients who can benefit from immunomodulation.
Up to 65% of patients with AML lack of molecular markers useful for follow-up. The Wims tumor gene (WT1) is overexpressed in more than 80% of AML patients at diagnosis, hence its utility as MRD marker in these patients.
The objective of this study is to analyze the usefulness of WT1 expression for MRD study and relapse prediction in patients with AML after allogeneic SCT and to compare the results with those from flow cytometry and chimerism.
149 samples were studied (63 BM and 86 PB) from 11 patients with AML (table 1) lacking of other molecular markers at diagnosis, who underwent allogeneic SCT in a single center from 2003 and 2008. Quantification of WT1 expression was performed by quantitative RT-PCR (LightCycler 1.2) using cDNA obtained from 1 ug of RNA (Trizol®, Invitrogen). K562 cell line was used as calibrator and ABL as reference genes (relative quantification). Cut-off values were defined at 0.5% in BM samples and 0.01% in PB. Results were correlated with those from flow cytometry and chimerism from the same samples.
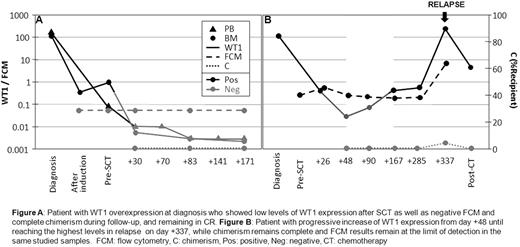
WT1 overexpression correlated with the presence of disease before transplantation (table 1). Post-transplantation, 5 of the 11 patients showed low and stable WT1 levels in BM and PB in the follow-up, remaining in CR (median follow-up 48 months, range 8-61). These 5 patients showed complete chimerism (CC) and negative MRD by flow cytomety (figure A). On the other hand, 6 from the 11 patients showed WT1 overexpression after SCT: 4 of them showed after initial low values, a significant increase in WT1 expression up to reaching positive levels in a median of 139 days (50-167), while 2 patients showed positive values continuously. Two patients showed positive levels only in PB samples, one only in BM and 3 both in BM and PB. From these 6 patients, 5 relapsed (1 with extramedullar disease who showed WT1 overexpression only in PB) in a median of 9 months after SCT (2.4-11). In one case, WT1 overexpression occured at the same time as relapse, while in the other 4 cases, relapse occured in a median of 137 days (134-170) after a significant increase in WT1 levels was seen in two consecutive samples. In these 5 relapsed patients, neither flow cytometry nor chimerism predicted relapse (figure B).
WT1 overexpression correlated with disease burden in AML patients after allogeneic SCT. In realapsed patients, relapse both medullar and extramedullar was anticipated sinificantly by WT1 overexpression compared to flow cytometry and chimerism. Quantification of WT1 overexpression by RT-PCR should be used for MRD detection in the follow-up after SCT in AML patients (not only in BM but also in PB for extramedullar disease detection) in order to facilitate immunossupresive therapy management and to select early DLI candidates.
Figure A Patient with WT1 overexpression at diagnosis who showed low levels of WT1 expression after SCT as well as negative FCM and complete chimerism during follow-up, and remaining in CR. Figure B: Patient with progressive increase of WT1 expression from day +48 untill reaching the highest levels in relapse on day +337, while chimerism remains complete and FCM results remain at the limit of delection in the same studied samples. FCM: flow cytometry, C: chimerism, Pos: positive, Neg: negative, CT: chemotherapy
Figure A Patient with WT1 overexpression at diagnosis who showed low levels of WT1 expression after SCT as well as negative FCM and complete chimerism during follow-up, and remaining in CR. Figure B: Patient with progressive increase of WT1 expression from day +48 untill reaching the highest levels in relapse on day +337, while chimerism remains complete and FCM results remain at the limit of delection in the same studied samples. FCM: flow cytometry, C: chimerism, Pos: positive, Neg: negative, CT: chemotherapy
No relevant conflicts of interest to declare.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal