Abstract
Abstract 1316
Poster Board I-340
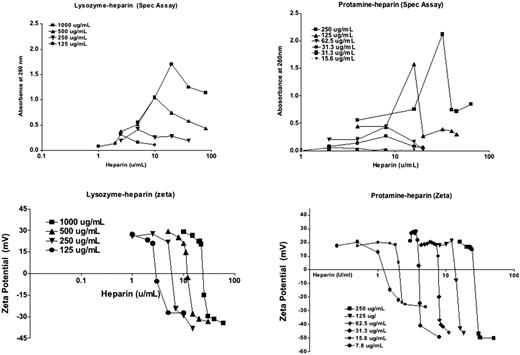
The immune response in Heparin-Induced Thrombocytopenia (HIT) is directed to and initiated by large multimolecular complexes of Platelet Factor 4 (PF4) and heparin. We, and others, have previously shown that PF4 and heparin multimolecular assembly occurs through colloidal interactions, wherein heparin, a negatively charged polymeric compound, facilitates macromolecular assembly by binding and neutralizing PF4's positive charge (Suvarna, Blood 2007). In these same studies, we also demonstrated that changes in the molar ratios of the two reactants result in PF4/heparin (PF4:H) complexes with markedly altered biophysical properties and immunogenicity. Because PF4:H electrostatic interactions are non-specific, we hypothesized that other positively charged proteins would exhibit similar colloidal interactions with heparin. To test this hypothesis, we selected two positively charged proteins (protamine and lysozyme) and studied heparin-dependent complex formation by spectrophotometry (A280nm), and zeta potential (Zeta Sizer, Malvern, UK). Protamine sulfate (250, 125, 62.5, 31.2, 15.6 and 7.8 mcg/mL; Mw 5.1kDa) and lysozyme (1000, 500, 250 and 125 mcg/mL; Mw 14.3kDa) were mixed with various heparin concentrations (0-160 U/mL; activity 140U/mg; Mw 12kDa) and biophysical properties characterized by both instruments. Both protamine and lysozyme showed heparin-dependent complex formation, with peak particle formation occurring over a range of heparin concentrations (2-25 U/mL ) for both compounds. For protamine, particle formation was maximal at protamine:heparin (Pr:H) molar ratios of ∼2.5-3:1, whereas lysozyme formed peak particles at lysozyme;heparin (Ly:H) molar ratios of ∼5:1 (See figure). As with PF4:H complexes, size of complexes was dependent on mass amounts of protamine or lysozyme, with particle size increasing or decreasing in proportion to the amounts of protamine or lysozyme available for complex formation. These findings indicate that heparin is capable of forming macromolecular complexes with other proteins through charge dependent interactions. Additional in vitro and in vivo studies are underway to determine if Pr:H or Lys:H complexes exhibit cross-reactivity with PF4/heparin antibodies and if complex formation is associated with immunological consequences.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal