Abstract
Abstract 120
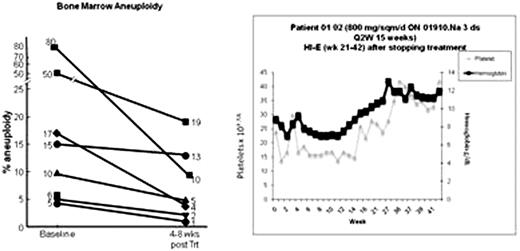
Patients with high risk MDS can be successfully treated with 5-azacytidine, or with lenalidomide but non-responding patients have few treatment options. Chemotherapy produces significant morbidity and very short remissions and most patients are too old for bone marrow transplantation. We previously demonstrated up-regulation of c-myc, survivin, and cyclin D1 in CD34+ cells in patients with trisomy 8 (and selected patients with monosomy 7). siRNA-mediated knockdown of survivin or c-myc decreased trisomy 8 cell growth in vitro (Sloand et al, Blood 2007, 110: 822). We postulated that increased cyclin D1 causes upregulation of survivin, resulting in resistance of these cells to apoptosis. The styryl sulfone, ON 01910.Na, decreases cyclin D1 accumulation in cultured bone marrow from patients with high risk trisomy 8 MDS and in some monosomy 7 patients (who also show upregulation of cyclin D1), while selectively decreasing blasts and aneuploidy with this cytogenetic abnormality (ASH Abstracts Nov 2008; 112: 1651). Here we examine the clinical response to ON1910 in an ongoing phase I/II clinical trial in which 13 evaluable patients with intermediate-1(int-1) to high risk MDS and treatment-refractory trisomy 8 AML were enrolled. Patients were treated with escalating doses of ON 01910.Na at 800 mg/m2 × 2 days every 3/4 weeks, 800 mg/m2 × 3 days every 2 weeks, 800 mg/m2 × 5 days every 2 weeks, and 1500 mg/m2 × 2 days every 3/4 weeks at two institutions. No significant toxicity could be ascribed to the drug. Patients with trisomy 8 and monosomy 7 demonstrated significant declines in aneuploidy measured by florescence in situ hybridization (FISH) (mean aneuploidy; 50% before and 24% after 1 cycle of treatment; p=0.02 :Fig below). Rather than becoming cytopenic, many patients showed substantial improvements of blood counts and one patient (01-02; graphic shown below) became red cell transfusion-independent and maintains his remission 14 months after stopping therapy. Cyclin D1 measurement by flow cytometry showed decreases of this protein in both CD34 and CD33 cells during infusion of ON 1910 infusion (example shown in Fig below). Results from individual evaluable patients are shown in table 1. These results indicate that modulation of cell cycle control by cyclin D1 may represent a novel targeted approach for trisomy 8 and monosomy 7 MDS.
| Protocol . | PID . | ON 01910.Na . | IPSS Pre-Trt . | Weeks on study . | Cytogenetics . | Prior 5-Aza . | % Blasts BM . | HI . | Survival weeks . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| Dosage mg/m2/24h | Pre-Rx | Week 4-8 F-Up | ||||||||
| 07-H-0225 | 01-01 | 800/72h Q2W | High | 10 | +8, -7, 5q- | Yes | 17 | 4 | N,P | 19 |
| 01-02 | 800/72h Q2W | High | 16 | −7 | No | 5 | 2 | E | 56+ | |
| 01-03 | 800/72h Q2W | High | 8 | −7, +8 | No | 10 | 5 | N | 52+ | |
| 02-01 | 800/120h Q2W | High | 6 | +8 | No | 50 | 19 | P,E | 26 | |
| 02-02* | 800/120h Q2W | High | 5 | +8/Complex | Yes | 15 | 13 | 33 | ||
| 02-03 | 800/120h Q1W | AML | 2 | +8 | No | 80 | 10 | 34 | ||
| 02-04 | 800/120h Q2W | High | 14 | t (3,21) | No | 5 | 1 | N | 21+ | |
| 02-05 | 800/120h Q2W | AML | 2 | +8 complex | No | 30 | ND | 2 | ||
| 02-06 | 800/120h Q2W | High | 4+ | +11 | No | 6 | 62 | 4+ | ||
| 04-15 | 01-04 | 800/48h 3/4 wks | High | 19+ | +14 | Yes | 25 | 30 | N | 19+ |
| 01-08 | 800/48h 3/4 wks | High | 17+ | Complex | No | 25 | 11 | 17+ | ||
| 01-09 | 800/48h 3/4 wks | Int-1 | 17+ | Normal | No | 12 | 15 | E | 17+ | |
| 01-11 | 1500/48h 3/4 wks | Int-2 | 15+ | 5q-/7 abn | No | 9 | 5 | 15+ | ||
| 01-12 | 1500/48h 3/4 wks | Int-1 | 15+ | ND | No | 10 | 2 | 15+ | ||
| 01-13 | 1500/48h 3/4 wks | Int-2 | 10 | +8/+13 | Yes | 15 | 20 | 13 |
| Protocol . | PID . | ON 01910.Na . | IPSS Pre-Trt . | Weeks on study . | Cytogenetics . | Prior 5-Aza . | % Blasts BM . | HI . | Survival weeks . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| Dosage mg/m2/24h | Pre-Rx | Week 4-8 F-Up | ||||||||
| 07-H-0225 | 01-01 | 800/72h Q2W | High | 10 | +8, -7, 5q- | Yes | 17 | 4 | N,P | 19 |
| 01-02 | 800/72h Q2W | High | 16 | −7 | No | 5 | 2 | E | 56+ | |
| 01-03 | 800/72h Q2W | High | 8 | −7, +8 | No | 10 | 5 | N | 52+ | |
| 02-01 | 800/120h Q2W | High | 6 | +8 | No | 50 | 19 | P,E | 26 | |
| 02-02* | 800/120h Q2W | High | 5 | +8/Complex | Yes | 15 | 13 | 33 | ||
| 02-03 | 800/120h Q1W | AML | 2 | +8 | No | 80 | 10 | 34 | ||
| 02-04 | 800/120h Q2W | High | 14 | t (3,21) | No | 5 | 1 | N | 21+ | |
| 02-05 | 800/120h Q2W | AML | 2 | +8 complex | No | 30 | ND | 2 | ||
| 02-06 | 800/120h Q2W | High | 4+ | +11 | No | 6 | 62 | 4+ | ||
| 04-15 | 01-04 | 800/48h 3/4 wks | High | 19+ | +14 | Yes | 25 | 30 | N | 19+ |
| 01-08 | 800/48h 3/4 wks | High | 17+ | Complex | No | 25 | 11 | 17+ | ||
| 01-09 | 800/48h 3/4 wks | Int-1 | 17+ | Normal | No | 12 | 15 | E | 17+ | |
| 01-11 | 1500/48h 3/4 wks | Int-2 | 15+ | 5q-/7 abn | No | 9 | 5 | 15+ | ||
| 01-12 | 1500/48h 3/4 wks | Int-1 | 15+ | ND | No | 10 | 2 | 15+ | ||
| 01-13 | 1500/48h 3/4 wks | Int-2 | 10 | +8/+13 | Yes | 15 | 20 | 13 |
BM=Bone Marrow; h=hours; wks=weeks; Q2W=every other week; SD=Stable disease; PD=Progressive disease; HI=Hematological Improvement (P=Platelet; E=Erythroid; N=Neutrophil): ND not done
Patient did not complete two infusions because of access problems
Sloand:Onconova: Research Funding. Olnes:Onconova: Research Funding. Galili:Onconova: Research Funding. Wilhelm:Onconova: Employment. Groopman:Onconova: Membership on an entity's Board of Directors or advisory committees. Raza:Onconova: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal