Abstract
Abstract 1178
Poster Board I-200
High-dose chemotherapy with autologous peripheral blood stem cell transplantation (PBSCT) has been shown to be of value in selected patients with malignant lymphoma and multiple myeloma (MM). Both granulocyte macrophage colony stimulating factor (GMCSF) and granulocyte colony stimulating factor (GCSF) are widely used following stem cell transplantation to support accelerated myeloid recovery and potentially shorten hospital stay. In the recent years, PBSCT is increasingly done in an ambulatory care setting and it is not clear if routine growth factor use has any potential benefit. We undertook this study to compare engraftment kinetics, incidence of febrile episodes, hospitalization duration and other outcomes among transplants done with or without growth factor support
We included adult patients undergoing PBSCT for myeloma and lymphoma between May 2007 and June 2009 in an ambulatory care setting. Prior to April 2008 (Group-1) all patients received GMCSF 500 mcg/ day starting on day 6 routinely. Since April 2008, there was a shift in clinical practice and routine use of GMCSF was discontinued due to lack of clear evidence regarding benefit. Patients transplanted between April 2008 and June 2009 did not routinely receive GMCSF (Group-2). Typical conditioning regimen for lymphoma was BEAM (BCNU, Etoposide, Cytosine Arabinose, Melphalan) and for multiple myeloma was melphalan 200mg/m2. Patients' demographics details, indication for PBSCT, duration of neutropenic fever, hospital stay, and neutrophil and platelet engraftment were collected from electronic medical records. Neutrophil engraftment is defined as the first day of 2 consecutive days when ANC was > 0.5 × 109/L and platelet engraftment is defined as first of 7 consecutive days on which patient's unsupported platelet count was greater than 20× 109/L.
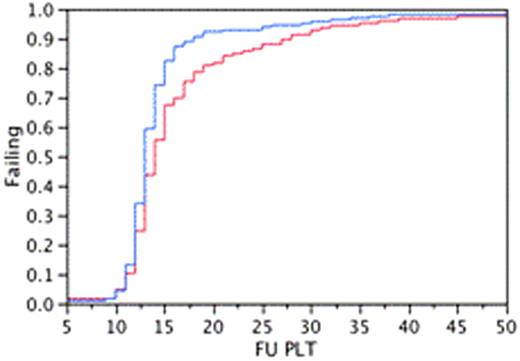
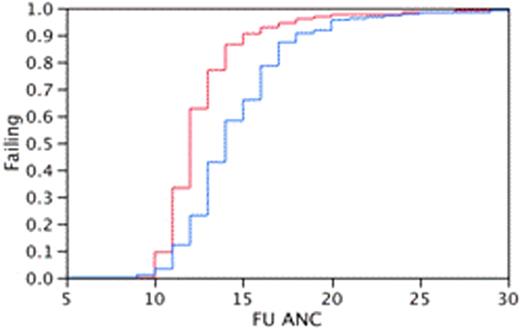
A total of 309 were included in this study, 126 (40%) patients in group-1 (69 with MM, 57 with lymphoma) and 183 (60%) in group-2 (100 with MM, 83 with lymphoma). In group-1, 73 (58%) and in group-2 130 (71%) were male patients. Median age was 59 (29-74) years in group 1 and 58 (24-76) years in group-2. Patients in group 1 received a median of 3.65 (1.1-8.7) versus 4.37 (1.1-9.54) CD34+ x106/kg in group 2 (P =0.005). Median time to neutrophil engraftment was 12 (95% CI 12, 12) for group-1 and 14 (95% CI; 13-14) for group-2 (Kaplan Meier analysis; log rank P < 0.0001). Median estimated time to platelet engraftment was 14 days (95% CI; 13, 15) for group 1 and 13 days (95% CI; 13-13) for group 2 (Kaplan Meier analysis; log rank P 0.007). In group-1 98 (78%) patients developed febrile episodes compared to 117(64%) in group-2 (P = 0.009). Median duration of fever was 2 days (range, 1-8) in group-1 compared to 1 day (range, 1-8) in group-2 (P = 0.0003). The median (range) duration of hospital stay was 7 (1-40) days for group-1 compared to 8 (2-56) days for group-2 (P=NS). There was no significant difference in mortality between these two groups.
Routine use of granulocyte growth factors is associated with a shorter time to neutrophil engraftment, but associated with a delay in platelet engraftment. The longer time to neutrophil engraftment without growth factor use does not translate into any increase in frequency of febrile episodes. On the contrary, higher number of febrile days is seen with use of growth factors, likely explained on the basis of drug fever. No difference in the frequency of engraftment syndrome or treatment related mortality was seen.
Kaplan Meier Analysis on Neutrophil and Platelet Engraftment Between Group1and Group 2
Kaplan Meier Analysis on Neutrophil and Platelet Engraftment Between Group1and Group 2
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal