Abstract
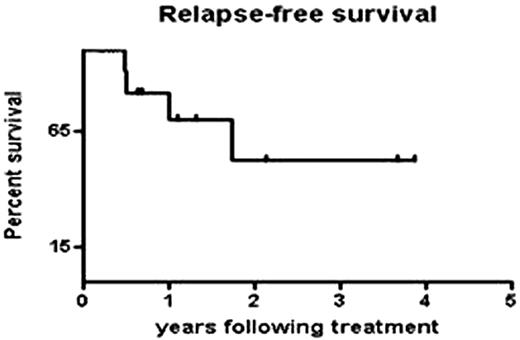
Myelodysplastic syndromes (MDS) are a diverse group of disorders characterized by cytopenias and ineffective hematopoiesis. Experimental evidence links bone marrow failure in a portion of MDS patients to a T cell-dominated autoimmune process. Immunosuppressive therapy (IST) can improve cytopenias in selected patients. Features associated with responsiveness to horse ATG in MDS are young age, low IPSS score, and the presence of HLA DR15 (Sloand E et al J Clin Oncol 2008 26(15):2505-11). The addition of cyclosporine to ATG improves response but continued use of this drug is associated with nephrotoxicity. Alemtuzumab, an anti-CD52 antibody, produces more profound lasting immunosuppression compared to ATG that is associated with transient lymphopenia. We conducted a non-randomized, off label, pilot, Phase II study of alemtuzumab (Campath®) in MDS patients who fit the criteria of those likely to respond to IST (Saunthararajah Y et al Blood. 2003 102(8):3025-7). Consecutive patients fulfilling the inclusion criteria received alemtuzumab 10mg IV for 10 days. Primary endpoints were changes in peripheral blood counts (platelets, absolute neutrophil count, reticulocyte count, hemoglobin). Secondary endpoints (in transfusion-dependent patients) included improvement in the transfusion requirements, duration of response, and late effects of treatment, relapse and survival. Median follow-up time was 11 months (range 1-46 months). Fifteen of 16 (93%) int-1 patients and 2 of 5 (40%) int-2 patients responded by 3 months following infusion. All responders were transfusion independent. Five of seven patients with abnormal cytogenetics at start of treatment had complete cytogenetic remission. One patient with monosomy 7 (constituting 65% of his bone marrow mononuclear cells by FISH) had a complete cytogenetic remission that has lasted 3.8 years thus far. Five of 9 (55%) patients evaluable at 9 months had completely normal blood counts. One patient developed immune thrombocytopenic purpura responsive to rituximab. Two int-2 patients developed leukemia and an additional int-2 patient with a history of smoking succumbed to small cell lung cancer. One death occurred in a non-responding int-1 patient due to disease progression. Two patients had declines in their counts (but not to pretreatment levels) and both responded to CsA. We monitored blood for EBV and CMV reactivation by polymerase chain reaction (PCR) weekly and 4 of the 22 patients became transiently positive for EBV but none developed disease and none of our patients had a significant infection while on drug. The response rate of int-1 patients at 3 months was superior to our previous study using ATG (53%; p=0.0071) but comparable to our results with ATG and CsA (93% p=1.0). Relapse was defined as requirement for additional treatment including cyclosporine; relapse-free survival of responders did not differ from those given ATG or ATG and CsA. The results from this pilot study indicate that Alemtuzumab is highly effective in producing durable responses in selected patients with int-1 MDS.
No relevant conflicts of interest to declare.

This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal