Abstract
Results of heavy-water labeling studies have challenged the notion that chronic lymphocytic leukemia (CLL) represents an accumulation of noncycling B cells. We examined leukemia cell turnover in Eμ-TCL1 transgenic (TCL1-Tg) mice, which develop a CLL-like disease at 8 to 12 months of age. We found that leukemia cells in these mice not only had higher proportions of proliferating cells but also apoptotic cells than did nonleukemic lymphocytes. We crossed TCL1-Tg with BAFF-Tg mice, which express high levels of CD257. TCL1×BAFF-Tg mice developed CLL-like disease at a significantly younger age and had more rapid disease progression and shorter survival than TCL1-Tg mice. Leukemia cells of TCL1×BAFF-Tg mice had similar proportions of proliferating cells, but fewer proportions of dying cells, than did the CLL cells of TCL1-Tg mice. Moreover, leukemia cells from either TCL1×BAFF-Tg or TCL1-Tg mice produced more aggressive disease when transferred into BAFF-Tg mice than into wild-type (WT) mice. Neutralization of CD257 resulted in rapid reduction in circulating leukemia cells. These results indicate that the leukemia cells of TCL1-Tg mice undergo high levels of spontaneous apoptosis that is offset by relatively high rates of leukemia cell proliferation, which might allow for acquisition of mutations that contribute to disease evolution.
Introduction
Chronic lymphocytic leukemia (CLL), the most common adult leukemia in Western countries, is a disease of neoplastic, monoclonal CD5+ B cells, which accumulate in the blood, marrow, and secondary lymphoid tissues. For years this disease has been viewed as a prime example of a tumor with very low levels of cell turnover in which intrinsic resistance to apoptosis was responsible for a gradual accumulation of neoplastic CD5+ B cells.1 Consistent with this notion, CLL cells express high levels of antiapoptotic Bcl-2 family members as well as inhibitors of apoptosis proteins.2-5
However, recent studies have challenged this view. CLL cells were found to undergo spontaneous apoptosis in vitro under conditions that could support growth of established B-cell lines.6 Spontaneous apoptosis of CLL cells could be inhibited by accessory cells in the leukemia microenvironment, such as mesenchymal-derived marrow stromal cells,7 follicular dendritic cells,8 or nurselike cells (NLCs),6,9-11 suggesting that CLL cells do not have an intrinsic resistance to apoptosis. In particular, NLCs produce factors that can enhance leukemia cell survival in vitro.6,12 Among these factors is a member of the tumor necrosis factor family, most notably CD257 (B cell–activating factor of tumor necrosis factor family [BAFF], also called tumor necrosis factor and apoptosis ligand-related leukocyte-expressed ligand 1 or B-lymphocyte stimulator).10 CLL B cells express 3 receptors for CD257, namely CD269 (formerly called B-cell maturation antigen), CD267 (formerly called transmembrane activator and CAML interactor), and CD268 (formerly called BAFF receptor, or BR3).13,14 Interaction of CD257 with these receptors can enhance leukemia cell survival in vitro, a mechanism that potentially could contribute to disease progression in vivo.14 The existence of cycling cells in CLL patients is suggested by recent studies using heavy water to label leukemia cells in vivo.15,16 These studies demonstrated that some patients with apparently indolent disease might generate up to 1% of their entire leukemia cell population each day, implying that in CLL there might be high rates of cell turnover that counterbalance such high rates of leukemia cell proliferation. As such, we hypothesize that B-cell survival factors such as CD257 could enhance disease progression merely by inhibiting leukemia cell turnover, thereby allowing for enhanced accumulation of nascent leukemia cells in vivo.
We examined for this in an animal model for CLL, namely mice made transgenic (Tg) for the human T-cell leukemia 1 (TCL1) gene controlled by the immunoglobulin μ heavy-chain promoter/enhancer, known as Eμ-TCL1-Tg mice (here noted as TCL1-Tg mice). Such animals have constitutive high-level expression of Tcl1 in mature B cells. At 8 to 12 months of age, these mice develop a CLL-like disease that shares many features with human CLL, such as excessive accumulation of monoclonal CD5+ B cells in blood and lymphoid tissues.17 Originally identified in T-cell leukemia, Tcl1 enhances Akt kinase activity, mediates its nuclear translocation, and influences cell activation and survival.18 More recently it was shown that Tcl1 is expressed by human CLL cells and also interacts with c-Jun, JunB, and c-Fos to inhibit activator protein 1 transcriptional activity. Furthermore, Tcl1 was found to play a role in the activation of nuclear factor κB (NF-κB) by interacting with p300/cyclic adenosine monophosphate response element binding protein.19 Because the leukemia cells that develop in TCL1-Tg mice express high levels of Tcl1, it was assumed that these cells had low rates of spontaneous apoptosis, in contrast to what now is speculated to occur in at least some patients with CLL. In our study, we examined whether the leukemia cells of TCL1-Tg mice instead undergo high rates of cell turnover and whether CD257 could suppress spontaneous apoptosis of leukemia cells, thereby offsetting cell turnover in favor of disease development and progression in this animal model of CLL.
Methods
Mice
Eμ-TCL1 mice (TCL1-Tg mice) were backcrossed for at least 9 generations onto the C57Bl/6 background. BAFF-Tg mice were from L. Gorelik (Biogen Idec). All mice were housed under conventional barrier protection in accordance with University of California San Diego and National Institutes of Health guidelines, and mouse protocols were approved by the University of California San Diego Institutional Animal Care Committee. Survival data were obtained by observing cohorts of 12 mice (6 females and 6 males) of each genotype.
Adoptive cell transfer
For adoptive transfer, splenic cells from TCL1XBAFF-Tg mice were labeled with CD5-allophycocyanin and CD3–fluorescein isothiocyanate (FITC) antibodies and separated using a FACSAria cell sorter (Becton Dickinson). CD3−CD5+ sorted cells (106) in 100 μL of phosphate-buffered saline (PBS) were injected retro-orbitally into either wild-type (WT) or BAFF-Tg mice that were gamma-irradiated with 6 Gy rad and anesthetized using isoflurane (Abbott Labs).
Leukocyte preparation
Blood was collected retro-orbitally with a capillary tube into ethylenediaminetetraacetic acid–coated blood collection tubes (Becton Dickinson). Single-cell suspensions were obtained from spleen, lymph nodes (axillary, brachial, and inguinal or mesenteric), and thymus by grinding through nylon sieves (BD Falcon). For some experiments, B cells were positively enriched from WT mice splenocytes with B220 magnetic-activated cell sorting (MACS) beads and leukemic cells were negatively enriched with CD3 MACS beads, followed by positive selection with CD5 MACS beads (Miltenyi Biotec) according to manufacturer's protocol.
Flow cytometry
Single-cell suspensions were stained for surface expression with phycoerythrin (PE)–labeled anti-CD5, allophycocyanin-labeled anti-B220 (CD45R), and FITC-labeled anti-CD3 antibodies (Pharmingen). For intracellular staining, cells were stained for surface markers before fixation and permeabilization using the Fix & Perm kit (Caltag) and staining with a PE-labeled monoclonal human TCL1 antibody (Pharmingen). Propidium iodide was used to exclude dead cells. Flow cytometry analyses were performed with either BD FACSCalibur or Accuri C6 flow cytometers. Plots were done with FlowJo software (TreeStar).
In vitro apoptosis measurements
Purified B cells from 12-month-old WT control mice and CD5+ leukemic cells from TCL-Tg mice were treated with 200 ng/mL recombinant human CD257 (rhCD257; a kind gift from Dr G. Zhang, National Jewish Medical and Research Center) for the indicated time periods and terminal deoxynucleotidyl transferase deoxyuridine-triphosphatase nick-end labeling (TUNEL) assays were performed with In Situ Cell Death Detection Kit TMR Red (Roche) and analyzed with an Accuri C6 flow cytometer. Cell viability was also examined by trypan blue exclusion.
TUNEL and CD5 staining on frozen splenic sections
Frozen spleen sections were fixed and permeabilized with Perm/Fix solution (BD Biosciences) according to instruction. TUNEL staining was performed as described above and washed 3 times with PBS, followed by CD5-biotin and streptavidin-FITC staining after blocking with 2% goat serum, 1% fetal calf serum, and 1% bovine serum albumin in PBS. Slides were counterstained with 4,6 diamidino-2-phenylindole before mounting with antifade solution.
Cell fractionation and immunoblots
Cytosolic and nuclear fractions were prepared as follows: 106 B cells or leukemic cells were treated with 200 ng/mL CD257 or control vehicle for the indicated periods. Cells were collected and suspended in buffer A (10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.8; 1.5mM MgCl2; 10mM KCl; 0.5mM dithiothreitol, 0.05% Nonidet P-40, and protease inhibitor cocktails from Roche) for 30 minutes on ice; nuclei and cell debris were subjected to 500g for 10 minutes at 4°C and supernatants (cytosol) were collected. Nuclear pellets were suspended in buffer B (5mM N-2-hydroxyethylpiperazine-N′;-2-ethanesulfonic acid, pH 7.8; 1.5mM MgCl2; 0.2mM ethylenediaminetetraacetic acid, 0.5mM dithiothreitol, 26% glycerol, and protease inhibitors cocktail). Cytosolic and nuclear fractions were gel separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis for immunoblot analyses with antibodies specific for: Bcl-2, Bcl-XL, RelB, p65, histone deacetylase 1 (HDAC1; Santa Cruz Biotechnology), A1/Bfl1, Mcl-1 (Cell Signaling Technology), α-tubulin (Sigma-Aldrich), or p52 (Upstate Biotechnology).
Cell proliferation assays
For in vivo 5-bromo-2-deoxyuridine (BrdU) labeling, 10 mg/mL BrdU (BD Biosciences) in PBS was injected intraperitoneally and 12 hours later splenocytes were collected and stained with anti–BrdU-FITC antibody (Pharmingen) after fixation with 2% paraformaldehyde, permeabilization using 3M HCl plus 2% Tween 20 solution, and neutralization with 1M boric acid. For in vitro Ki-67 staining, 106 cells were surface labeled with CD5-PE antibody. Labeled cells were permeabilized with HCI plus Tween and labeled with Ki-67 antibody (Pharmingen).
Decoy receptors CD268-Fc and CD269-Fc
Decoy receptors CD268-Fc and CD269-Fc were kindly provided by Genentech and Biogen Idec, respectively.
Southern blot analysis
Splenic leukemia cells were processed to single cells and cryopreserved. DNA was isolated and digested with HindIII and analyzed by Southern blotting using a JH-probe as described.20 Detection limit of was around 20% of clonal expansion, as determined by serial dilutions of hybridoma-derived DNA with genomic DNA of NIH3T3 cells.
Statistical analysis
Student t tests assuming equal variance were performed for most of our studies. For comparison of TUNEL-positive cells in splenic sections of the 4 different mice strains, a 1-way, repeated measure using analysis of variance with Bonferroni correction was performed. For survival curve shown by Kaplan-Meier plot, log-rank (Mantel-Cox) test and log-rank test for trend were performed with Prism V.5 software (GraphPad). P values are indicated in the figures.
Results
CD5+B220low B cells in TCL1-Tg mice do have a high proliferation rate
In comparison with normal B cells, leukemic cells of TCL1-Tg mice express less B220 but are positive for the T-cell marker CD5, which is not expressed normally by mature B cells. These features are similar to the expression patterns of CD5 and CD20 in human CLL.17,21 To test whether the proliferation rate of leukemic B220lowCD5+ cells of TCL1-Tg mice is different from normal B cells we injected 5-bromo-2-deoxyuridine (BrdU) into 4- to 9-month-old TCL1-Tg mice and into WT littermates. We measured BrdU incorporation of CD5−B220+, CD5+B220low, and CD5+B220− cells by flow cytometry. We found that BrdU incorporation into leukemic cells that represented cells entering S phase was significantly higher than BrdU incorporation into normal B or T cells (Figure 1A-B). Surprisingly, BrdU incorporation into normal B or T cells did not differ from TCL1-Tg mice and WT mice. This may explain why young TCL1-Tg mice have normal B- and T-cell counts. BrdU incorporation correlated well with expression of the proliferation marker Ki-67 that was enhanced in leukemic cells of TCL1-Tg mice (Figure 1B-C; supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Assuming that the leukemic cells proliferate constantly at this high rate cell counts are expected to double every 2 to 3 weeks. However, this was by far not the case, and disease development observed in TCL1-Tg mice was much slower. Consequently, a substantial amount of leukemic cells must undergo cell death. Indeed, there is a much higher death rate of CD5+B220+ cells in spleens TCL1-Tg mice than of normal CD5−B220+ B cells, as measured by terminal deoxynucleotidyl transferase deoxyuridine-triphosphatase nick end labeling (TUNEL) and costained with either CD5-FITC or CD45R-FITC (Figure 1D-E; supplemental Figure 1B-C). Thus, although cell proliferation of leukemic cells in TCL1-Tg mice is high, a substantial part of leukemic cells underwent apoptosis, slowing down disease progression. Taken together, we found that CLL cell turnover in TCL1-Tg mice is unexpectedly high, closely resembling in vivo kinetics of human CLL,15 which is a new and important feature of the TCL1-Tg mouse model that is shared with human CLL disease.
CD5+B220low B CLL cells from TCL1-Tg mice have a high cell turnover. (A) In vivo cell proliferation of different cell populations of WT (top panel) or TCL1-Tg (bottom panels) mice was studied by injecting 10 mg/mL BrdU intraperitoneally into the mice strains indicated after analysis of splenic cells by flow 12 hours later using the antibodies indicated (n = 6 mice each strain). We gated on CD5+B220− T cells (P1), CD5−B220+ B cells (P2), or CD5+B220low B cells (P3), as indicated in the far left panels. We determined the proliferation rates for each of these subpopulations by evaluating for the proportion of cells that incorporated BrdU (rectangles), as indicated in the panels to the right. Each of these panels depicts the BrdU fluorescence of gated cells in P1, P2, or P3, respectively. (B) Statistical analysis of the results obtained in panel A. (C) CD5+B220low B CLL cells from TCL1-Tg were also tested for levels for Ki-67 using intracellular flow cytometry and those results were compared with B220+ splenic B cells from WT mice (n = 3 mice each strain). (D-E) Frozen splenic sections of mouse strains indicated were costained for CD5-FITC and TUNEL-TMR-Red. Data shown were average of TUNEL-positive cells/field from splenic sections (n = 6 mice). Magnification of the objective lense: 40×/1.3 Oil DIC. Camera: Zeiss AxioCam HR. Software: AxioVs40AC v4.5.0.0.
CD5+B220low B CLL cells from TCL1-Tg mice have a high cell turnover. (A) In vivo cell proliferation of different cell populations of WT (top panel) or TCL1-Tg (bottom panels) mice was studied by injecting 10 mg/mL BrdU intraperitoneally into the mice strains indicated after analysis of splenic cells by flow 12 hours later using the antibodies indicated (n = 6 mice each strain). We gated on CD5+B220− T cells (P1), CD5−B220+ B cells (P2), or CD5+B220low B cells (P3), as indicated in the far left panels. We determined the proliferation rates for each of these subpopulations by evaluating for the proportion of cells that incorporated BrdU (rectangles), as indicated in the panels to the right. Each of these panels depicts the BrdU fluorescence of gated cells in P1, P2, or P3, respectively. (B) Statistical analysis of the results obtained in panel A. (C) CD5+B220low B CLL cells from TCL1-Tg were also tested for levels for Ki-67 using intracellular flow cytometry and those results were compared with B220+ splenic B cells from WT mice (n = 3 mice each strain). (D-E) Frozen splenic sections of mouse strains indicated were costained for CD5-FITC and TUNEL-TMR-Red. Data shown were average of TUNEL-positive cells/field from splenic sections (n = 6 mice). Magnification of the objective lense: 40×/1.3 Oil DIC. Camera: Zeiss AxioCam HR. Software: AxioVs40AC v4.5.0.0.
CD257 increases survival of leukemic cells from TCL1-Tg mice
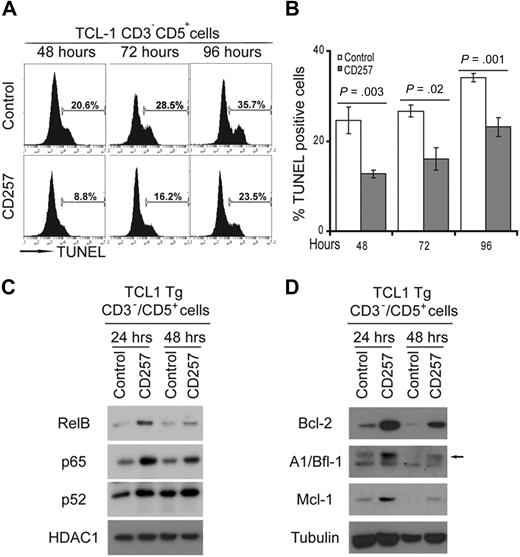
As we demonstrated earlier, CD257 is a potent survival factor for human CLL cells in vitro.10 We examined the effect of CD257 on CD3−CD5+ leukemic B cells from TCL1-Tg mice. We monitored for apoptosis of CD3−CD5+ B cells from TCL1-Tg mice (n = 3) that were cultured with or without recombinant human (rh) CD257, using TUNEL assay (Figure 2A-B; supplemental Figure 2). Culture with rhCD257 significantly reduced the proportion of cells undergoing apoptosis. As we showed previously, rhCD257 activated both classical and alternative NF-κB signaling in normal B cells and human CLL cells.14,22 Similarly, stimulation of normal or neoplastic B cells from WT or TCL1-Tg mice with rhCD257 resulted in their having increased amounts of nuclear p65/RelA, RelB, and p52 (Figure 2C). Correspondingly, stimulation of such B cells with rhCD257 induced increased expression of several NF-κB target genes, including those encoding the antiapoptotic proteins Bcl-2, Bcl-XL, Mcl-1, and A1/Bfl-1 (Figure 2D). Thus, rhCD257 acted in a very similar way in leukemic B cells from TCL1-Tg mice as it did in human CLL cells.
CD257 enhances survival of CD3−CD5+ CLL cells from TCL1-Tg mice and activates NF-κB. (A) CD3−CD5+ CLL cells from TCL1-Tg mice were cultured in absence (top line) or presence (bottom line) of 200 ng/mL rhCD257 and apoptosis was determined by TUNEL assay at different time points as indicated (n = 3 independent experiments). One representative assay is shown for each experiment. (B) Statistical analysis of data obtained in panel A. Error bars represent SD of 3 independent experiments. (C) Immunoblot from nuclear extracts of cells treated for different times with 200 ng/mL rhCD257, or left untreated, using antibodies to detect activation of the classical (p65) or alternative (RelB, p52) NF-κB pathway. HDAC1 served as a loading control. Shown is a representative blot (n = 3 independent experiments). (D) Immunoblot of cytosolic extracts of the same cells used in panel C immunoblotted for the antibodies indicated. Tubulin was used as a loading control. Shown is a representative blot (n = 3 independent experiments).
CD257 enhances survival of CD3−CD5+ CLL cells from TCL1-Tg mice and activates NF-κB. (A) CD3−CD5+ CLL cells from TCL1-Tg mice were cultured in absence (top line) or presence (bottom line) of 200 ng/mL rhCD257 and apoptosis was determined by TUNEL assay at different time points as indicated (n = 3 independent experiments). One representative assay is shown for each experiment. (B) Statistical analysis of data obtained in panel A. Error bars represent SD of 3 independent experiments. (C) Immunoblot from nuclear extracts of cells treated for different times with 200 ng/mL rhCD257, or left untreated, using antibodies to detect activation of the classical (p65) or alternative (RelB, p52) NF-κB pathway. HDAC1 served as a loading control. Shown is a representative blot (n = 3 independent experiments). (D) Immunoblot of cytosolic extracts of the same cells used in panel C immunoblotted for the antibodies indicated. Tubulin was used as a loading control. Shown is a representative blot (n = 3 independent experiments).
CD257 accelerates leukemogenesis in TCL1-Tg mice
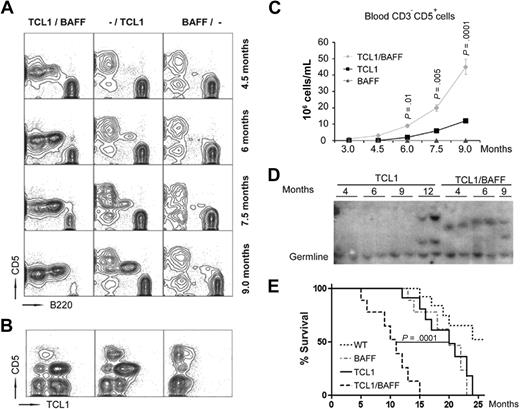
We crossed TCL1-Tg mice17 with BAFF-Tg mice23 and monitored cohorts of WT, BAFF-Tg, TCL1-Tg, or TCL1×BAFF-Tg mice for development of CLL-like disease (6 males and 6 females each cohort). Whereas TCL1-Tg mice develop a leukemic CD5+B220low population at approximately 7 months of age, TCL1×BAFF-Tg mice develop such a population before 4.5 months of age (Figure 3A). We did not observe a sex bias in disease development. WT or BAFF-Tg mice did not develop a leukemic cell population throughout their life spans. The CD5+ B cells expressed human TCL1 (hTCL1), as assessed by intracellular flow cytometry (Figure 3B). At 9 months of age the mean blood count of CD5+ B cells in TCL1×BAFF-Tg mice (45 × 106 cells/mL) was significantly greater than that of TCL1-Tg mice (10 × 106 cells/mL; Figure 3C). In accordance with this, blood smears of 9-month-old mice revealed that both TCL1-Tg and TCL1×BAFF-Tg mice had mononuclear cells that predominately were mature lymphocytes with numerous cells appearing similar to smudge cells typically identified on blood smears of CLL patients (supplemental Figure 3).24 Southern blot analyses of splenocytes harvested at various ages detected oligoclonal, or monoclonal, splenic CLL cell populations already by 4 months of age in all TCL1×BAFF-Tg mice, whereas such cells could not be uniformly detected until 12 months of age in TCL1-Tg mice (Figure 3D). Collectively, these data indicated that TCL1×BAFF-Tg mice developed oligoclonal, or monoclonal, CD5+ B-cell lymphoproliferative disease resembling CLL much earlier than TCL1-Tg mice.
Constitutive CD257 production enhances leukemia development. (A) Flow cytometric analysis of blood cells from different mouse strains (TCL1×BAFF-Tg mice labeled as BAFF/TCL1; TCL1-Tg, as -/TCL1; and BAFF-Tg, as BAFF/-) collected at different ages as indicated and stained with CD5 and B220 antibodies. Each analysis was done in triplicate. One representative blot for each analysis is shown. (B) Representative contour plots of blood cells from 9-month-old mice (n = 3 in each cohort) stained with CD5 and hTCL1 antibodies. (C) Blood-derived CD3−CD5+ cells in the indicated mouse strains at different ages were measured by flow cytometry using CD3 and CD5 antibodies (n = 12 mice per cohort; error bars indicate SD). (D) Clonal expansion of TCL1-Tg and TCL1×BAFF-Tg mice splenocytes (n = 2 per cohort per indicated age) as determined by Southern blot analysis of genomic DNA using a JH-probe. (E) Survival (Kaplan-Meier) plots of cohorts of 12 age- and sex-matched mice of the indicated strains. Mean survival times were TCL1×BAFF-Tg mice, 11.2 ± 3.4; TCL1-Tg, 20.7 ± 3.9; and BAFF-Tg, 21.0 ± 3.8 months. More than 50% of WT mice were still alive at the end of the 25-month observation period.
Constitutive CD257 production enhances leukemia development. (A) Flow cytometric analysis of blood cells from different mouse strains (TCL1×BAFF-Tg mice labeled as BAFF/TCL1; TCL1-Tg, as -/TCL1; and BAFF-Tg, as BAFF/-) collected at different ages as indicated and stained with CD5 and B220 antibodies. Each analysis was done in triplicate. One representative blot for each analysis is shown. (B) Representative contour plots of blood cells from 9-month-old mice (n = 3 in each cohort) stained with CD5 and hTCL1 antibodies. (C) Blood-derived CD3−CD5+ cells in the indicated mouse strains at different ages were measured by flow cytometry using CD3 and CD5 antibodies (n = 12 mice per cohort; error bars indicate SD). (D) Clonal expansion of TCL1-Tg and TCL1×BAFF-Tg mice splenocytes (n = 2 per cohort per indicated age) as determined by Southern blot analysis of genomic DNA using a JH-probe. (E) Survival (Kaplan-Meier) plots of cohorts of 12 age- and sex-matched mice of the indicated strains. Mean survival times were TCL1×BAFF-Tg mice, 11.2 ± 3.4; TCL1-Tg, 20.7 ± 3.9; and BAFF-Tg, 21.0 ± 3.8 months. More than 50% of WT mice were still alive at the end of the 25-month observation period.
We found the life span of TCL1×BAFF-Tg mice was significantly shorter than that of TCL1-Tg or BAFF-Tg mice (Figure 3E). Whereas both TCL1×BAFF-Tg mice and TCL1-Tg mice primarily died of leukemia, most BAFF-Tg mice died of renal failure due to autoimmune disease, as previously noted.23 None of the TCL1×BAFF-Tg mice examined on necropsy had developed overt renal pathology, apparently because they had died too young for kidney disease manifestation. In summary, presence of extrinsic CD257 in TCL1-Tg mice led to a more aggressive disease course and shorter survival.
CD257 enhances survival rather than proliferation of leukemia cells
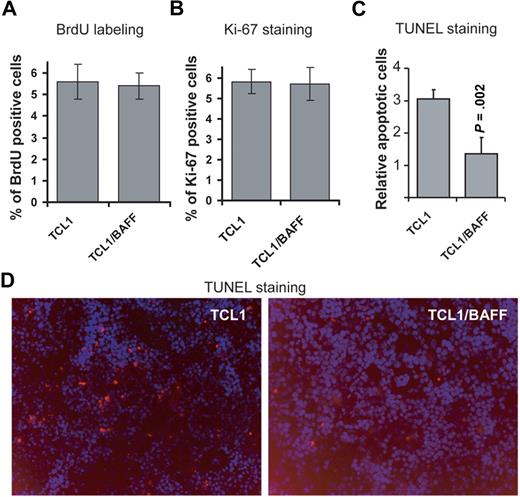
Because double-transgenic mice developed leukemia at a significantly younger age than TCL1-Tg mice, we investigated whether constant CD257 exposure increased the rate of leukemia cell proliferation by labeling cells in vivo with BrdU. Importantly, we did not observe a significant difference in BrdU incorporation by leukemia cells from TCL1-Tg compared with cells from TCL1×BAFF-Tg mice (Figure 4A, supplemental Figure 4A). Furthermore, the proportions of cells labeling with the proliferation marker Ki-67 were not different between leukemia cells from TCL1-Tg mice versus those obtained from TCL1×BAFF-Tg mice (Figure 4B, supplemental Figure 4B). TUNEL staining of spleen sections revealed that TCL1-Tg mice had 3 times more apoptotic cells in their spleen than TCL1×BAFF-Tg mice (Figure 4C-D), suggesting that CD257 promotes B-cell expansion mainly by inhibiting B-cell apoptosis in vivo.
CD257 does not affect cell proliferation but enhances survival of leukemia cells. (A) Proliferation of CD3−CD5+ lymphocytes obtained from TCL1-Tg or TCL1×BAFF-Tg mice was measured by BrdU incorporation. For this, mice were pulsed with BrdU and 12 hours later splenocytes were collected and stained with anti-BrdU. Mouse immunoglobulin G was used as an isotype control for anti-BrdU. Splenocytes from TCL1-Tg and TCL1×BAFF-Tg mice were gated for CD3−CD5+ and analyzed by flow using anti-BrdU. Results are from triplicate experiments (n = 3 mice each strain). (B) Ki-67 antibody stainings. Cells were obtained and gated as in panel A and stained for intracellular Ki-67 expression. Mouse immunoglobulin G was used as isotype control for anti–Ki-67. Results are from triplicate experiments (n = 3 mice each strain). (C-D) Frozen splenic sections of mouse strains indicated were stained for TUNEL-TMR-Red. Data shown were average of relative TUNEL-positive cells from splenic sections (n = 5 mice). Magnification of the objective lense: 20×/0.50 Camera: Zeiss AxioCam HR. Software: AxioVs40AC v4.5.0.0.
CD257 does not affect cell proliferation but enhances survival of leukemia cells. (A) Proliferation of CD3−CD5+ lymphocytes obtained from TCL1-Tg or TCL1×BAFF-Tg mice was measured by BrdU incorporation. For this, mice were pulsed with BrdU and 12 hours later splenocytes were collected and stained with anti-BrdU. Mouse immunoglobulin G was used as an isotype control for anti-BrdU. Splenocytes from TCL1-Tg and TCL1×BAFF-Tg mice were gated for CD3−CD5+ and analyzed by flow using anti-BrdU. Results are from triplicate experiments (n = 3 mice each strain). (B) Ki-67 antibody stainings. Cells were obtained and gated as in panel A and stained for intracellular Ki-67 expression. Mouse immunoglobulin G was used as isotype control for anti–Ki-67. Results are from triplicate experiments (n = 3 mice each strain). (C-D) Frozen splenic sections of mouse strains indicated were stained for TUNEL-TMR-Red. Data shown were average of relative TUNEL-positive cells from splenic sections (n = 5 mice). Magnification of the objective lense: 20×/0.50 Camera: Zeiss AxioCam HR. Software: AxioVs40AC v4.5.0.0.
Comparison of genome-wide gene expression of CD3−CD5+ splenic B cells from 12-month-old TCL1-Tg mice (n = 3) versus age-matched TCL1×BAFF-Tg mice (n = 3) showed similar expression levels of genes encoding proteins involved in cell proliferation (P = .39; supplemental Table 1). Taken together, these data suggest that CD257 does not promote leukemogenesis in TCL1-Tg mice by enhancing the rate of leukemia cell proliferation but rather by elongating their life span.
Transfer of CD3−CD5+ leukemic cells to BAFF-Tg mice results in rapid disease progression
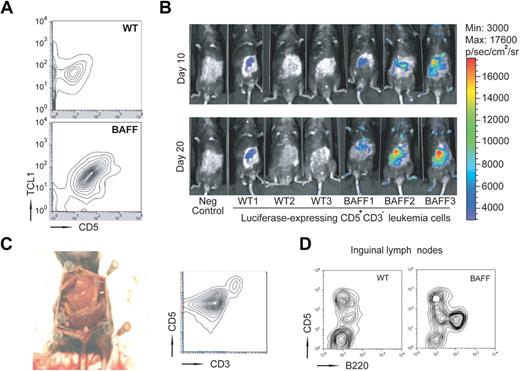
To exclude the possibility that TCL1×BAFF-Tg mice have intrinsic factor produced governing the disease course and to fully test our hypothesis that factors extrinsic to the leukemia cells play a prominent role in CLL development, we performed adoptive cell transfers of leukemic cells from TCL1×BAFF-Tg and from TCL1-Tg mice (data from TCL1-Tg mice not shown). For this, we isolated CD3−CD5+ CLL cells from spleens of 9-month-old TCL1×BAFF-Tg or TCL1-Tg mice, infused 106 such cells into either BAFF-Tg or WT mice, and monitored the growth of CD5+ hTCL1+ leukemia cells over time (Figure 5A). Whereas CD5+ hTCL1+ cells were undetectable in WT mice 30 days after adoptive transfer, such cells were readily identified in the blood of BAFF-Tg recipient mice at this time. To trace the leukemic cells after adoptive transfer, we transduced the CD3−CD5+ leukemic B cells with a luciferase-expressing lentivirus before injection. Bioluminescence-based imaging 10 and 20 days after adoptive transfer revealed accumulation of leukemia cells primarily in BAFF-Tg recipients, but not in WT recipients (Figure 5B). Necropsy at 6 months after adoptive transfer revealed that BAFF-Tg mice had massively enlarged spleens that were extensively infiltrated with leukemia cells, accounting for more than 90% of total splenocytes (Figure 5C). These mice also had enlarged lymph nodes that were heavily infiltrated with leukemic B cells as well (Figure 5D), but a normal-appearing liver and thymus. WT recipients, on the other hand, had normal-sized spleens with leukemic cells accounting for only 10% (10.4% ± 1%; n = 3) of all splenocytes (not shown). Our results indicated that extrinsic CD257 was able to greatly accelerate CLL disease course.
CLL cells expand faster in vivo in the presence of constantly elevated levels of CD257. (A) Splenocytes from TCL1×BAFF-Tg mice were labeled with CD3 and CD5 antibodies and CD3−CD5+ were enriched by cell sorting. Sorted leukemic cells (106) were injected into either BAFF-Tg or WT recipients (n = 6 each group) and their expansion was analyzed 30 days later by flow cytometry. (B) Expansion of transferred CD3−CD5+ cells transduced with a luciferase-expressing lentivirus before injection was measured by bioluminescence at the indicated time points. (C) Representative autopsy and flow cytometry of splenocytes from a BAFF-Tg mouse 5 months after adoptive transfer (n = 6). Camera: Nikon COOLPIX 995. (D) Representative flow cytometry of cells obtained from inguinal lymph nodes from WT or BAFF-Tg mice 5 months after adoptive transfer (n = 6 mice each group).
CLL cells expand faster in vivo in the presence of constantly elevated levels of CD257. (A) Splenocytes from TCL1×BAFF-Tg mice were labeled with CD3 and CD5 antibodies and CD3−CD5+ were enriched by cell sorting. Sorted leukemic cells (106) were injected into either BAFF-Tg or WT recipients (n = 6 each group) and their expansion was analyzed 30 days later by flow cytometry. (B) Expansion of transferred CD3−CD5+ cells transduced with a luciferase-expressing lentivirus before injection was measured by bioluminescence at the indicated time points. (C) Representative autopsy and flow cytometry of splenocytes from a BAFF-Tg mouse 5 months after adoptive transfer (n = 6). Camera: Nikon COOLPIX 995. (D) Representative flow cytometry of cells obtained from inguinal lymph nodes from WT or BAFF-Tg mice 5 months after adoptive transfer (n = 6 mice each group).
Inhibition of CD257-CD257 receptor interactions in vivo results in rapid decline of leukemic cell count
Incubation of B cells with soluble CD268-Fc decoy receptor completely abrogated the prosurvival effect of rhCD257 in vitro (Figure 6A). We next examined whether such decoy receptors could antagonize the effect of CD257 on leukemia B cells in vivo. To test this, we injected CD268-Fc or CD269-Fc decoy receptors,25,26 or control protein, into the peritoneum of BAFF-Tg mice that were previously adoptively transferred with CD3−CD5+ leukemic B cells from TCL1×BAFF-Tg mice and we monitored leukemia blood counts over time. Injection of 200 μg of CD268-Fc reduced the number of circulating CD3−CD5+ cells relative to that found in control-treated mice by almost 20% (18.2% ± 5.3%; n = 3) within 5 days (Figure 6B). Injection of 200 μg of CD269-Fc was even more effective in reducing the number of circulating CD3−CD5+ leukemia cells (37.4% ± 4.7%; n = 3). These findings indicate that strategies that target CD257 and/or CD257-expressing accessory cells might be effective in the treatment of CLL.
Blockade of CD257 induced the regression of leukemic cells from TCL1×BAFF-Tg mice grown in vitro and in BAFF-Tg mice. (A) In vitro viability of CD3−CD5+ cells from TCL1-Tg mice was examined by trypan blue exclusion in absence or presence of 200 ng/mL rhCD257 and absence or presence of 200 μg/mL decoy receptor CD268-Fc (n = 3). (B) BAFF-Tg mice inoculated with leukemic cells were injected with 200 μg of decoy receptors CD269-Fc or CD268-Fc. Peripheral CD3−CD5+ cell counts were determined 5 days after treatment with decoy receptors. Values represent relative mean ± SD CD3−CD5+ cell counts of treated mice compared with normalized actual CD3−CD5+ cell counts of untreated mice (n = 3 mice per each treatment group; each experiment was done in triplicate).
Blockade of CD257 induced the regression of leukemic cells from TCL1×BAFF-Tg mice grown in vitro and in BAFF-Tg mice. (A) In vitro viability of CD3−CD5+ cells from TCL1-Tg mice was examined by trypan blue exclusion in absence or presence of 200 ng/mL rhCD257 and absence or presence of 200 μg/mL decoy receptor CD268-Fc (n = 3). (B) BAFF-Tg mice inoculated with leukemic cells were injected with 200 μg of decoy receptors CD269-Fc or CD268-Fc. Peripheral CD3−CD5+ cell counts were determined 5 days after treatment with decoy receptors. Values represent relative mean ± SD CD3−CD5+ cell counts of treated mice compared with normalized actual CD3−CD5+ cell counts of untreated mice (n = 3 mice per each treatment group; each experiment was done in triplicate).
Discussion
A long-held view was that CLL represented an accumulation of slowly dividing, incompetent pathologic B cells that had an intrinsic resistance to apoptosis.1 However, recent studies have challenged this assumption, suggesting that CLL is a much more dynamic process in which relatively high rates of leukemia cell proliferation are offset by high rates of leukemia cell turnover.15 This different view of CLL may explain in part the activity of drugs in this disease that target cells undergoing replication.27 It also provides a model for the clonal evolution theory in which subclones of daughter cells are thought to establish predominance and possibly overtake the cell population if the subclones incur chance somatic mutations that provide them with a competitive advantage.28 Validation of this in future clinical studies would provide incentive for re-evaluating the current watch-and-wait strategies that withhold treatment for patients until they develop overtly progressive and/or symptomatic disease.29
Here we demonstrate that an animal model for CLL, the TCL1-Tg mouse, does indeed experience high rates of leukemia cell turnover in vivo and thus mimics human CLL in this important feature. This allows us to use this animal model to investigate the effect of factors that can offset or inhibit cell turnover on the pathogenesis and progression of this disease. It also allows for studies on the influence of factors that potentially could offset the balance between cell proliferation and survival that apparently governs the overall rate of disease progression.
We examined the effect of CD257 on this presumed balance. CD257 is a known survival factor for normal and leukemia B cells that is expressed at high levels by NLCs, which conceivably operate in the leukemia microenvironment to enhance leukemia cell survival in vivo.10 For this purpose, we used BAFF-Tg mice, which express high levels of CD257 in the liver, but also have elevated circulating CD257 that can cause systemic effects.23 Despite high-level expression of CD257, BAFF-Tg mice do not develop overt lymphoma per se, but rather systemic autoimmune disease.22,23 TCL1×BAFF-Tg mice, on the other hand, have continuous, high-level expression of Tcl1, and systemically elevated levels of CD257, which can promote leukemia cell survival. Consistent with the model that proposes this could offset the balance between leukemia cell proliferation and cell turnover. TCL1×BAFF-Tg mice more rapidly developed leukemia and had more aggresive disease than TCL1-Tg mice. The studies involving adoptive transfer of leukemia CD5+ B cells into WT versus BAFF-Tg mice provided further evidence that extrinsic CD257 plays a pathogenic role in leukemia progression. Whereas BAFF-Tg transplant recipient mice became sick relatively early due to rapidly progressive leukemia, WT recipients remained healthy for many months, experiencing only slow disease progression. This effect of CD257 could be inhibited by soluble CD268-Fc or CD269-Fc decoy receptors, which resulted in an immediate and substantial drop in leukemia cell blood counts of such mice.
The capacity of CD257 to enhance leukemia survival was associated with elevated expression of antiapoptotic proteins, whose genes are regulated in part by NF-κB. Whereas the TCL1 transgene had a relatively weak effect on NF-κB activation in CLL cells, we observed a synergistic effect in presence of CD257 on NF-κB activation in such cells. Similarly, although freshly isolated circulating human CLL cells exhibited constitutively activated NF-κB, which apparently could enhance survival and contribute to disease progression,30,31 microarray analyses demonstrated increased levels of expression of NF-κB target genes in CLL cells that resided within the leukemic cell microenvironment.32 These findings point to microenvironmental factors as key survival signals, closely resembling the role of cytokines produced by myeloid cells in solid malignancies.33-36 Our results fit well with the model proposing that factors produced in the microenvironment contribute to leukemic cell survival and hence disease progression. It seems likely that CD257, which is expressed at high levels by NLCs within the leukemia microenvironment of lymphoid tissues,10 is one such factor that contributes to disease progression of human CLL.
Mouse models of human cancers can be used to investigate the effectiveness of therapeutic approaches before human clinical trials. We found relatively rapid leukemia cell turnover in TCL1-Tg mice, making this mouse model one with which to study the influence of factors or agents that alter either cell proliferation or cell death. Moreover, the adoptive transfer of leukemia cells into BAFF-Tg mice provides an excellent model with which to test agents that can interfere with signaling pathways that promote leukemia cell survival. Collectively, our findings reported here suggest that agents that target proliferating cells might work synergistically with agents that inhibit the survival factors in eliminating the disease. Thus, future treatment strategies should envelop consideration of the potentially dynamic nature of CLL so as to provide the most effective therapy for this disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr G. Zhang (National Jewish Medical and Research Center) for the kind gift of rhCD257. We thank Genentech and Biogen-Idec for the kind gifts of CD268-Fc and CD269-Fc, respectively, and Dr Laura Z. Rassenti for technical support, Dr Catriona Jamieson for providing the lentiviral construct, and Dr Sonja Jain for doing the statistics.
This project was funded by a Leukemia & Lymphoma Society Specialized Center of Research (SCOR) grant. A.P.K. is personally supported by a “Veni” grant from The Netherlands Organization for Health Research and Development. W.Z. is personally supported by a postdoctoral fellowship from Susan G. Komen for the Cure.
Authorship
Contribution: T.E. and A.P.K. designed the research, performed most of the experiments, analyzed the data, and wrote the manuscript; W.Z. designed the research, performed significant experiments, and analyzed the data; G.F.W. contributed to the design of the study and performed some in vitro experiments; H.Y.C. and J.L. performed some in vitro experiments; E.A. performed some in vitro experiments and provided help with mouse work; C.M.C. significantly contributed to the design of the study; M.K. designed the research; and T.J.K. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas J. Kipps, 3855 Health Sciences Dr, Rm 4307, San Diego, CA 92093-0820; e-mail: tkipps@ucsd.edu.
References
Author notes
*T.E., A.P.K., and W.Z. shared first authorship.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal