Abstract
Sepsis and septic shock remain an important medical problem, emphasizing the need to identify novel therapeutic opportunities. Hypovolemic hypotension, coagulation dysfunction, disturbed microcirculation, and multiorgan failure resulting from vascular leakage are often observed in these severe conditions. In the present study, we find that HKH20, a peptide derived from human high molecular weight kininogen (HK), down-regulates inflammatory reactions caused by Streptococcus pyogenes in a mouse model of sepsis. HK is a component of the pro-inflammatory and pro-coagulant contact system. Activation of the contact system in the bloodstream by S pyogenes leads to massive tissue damage in the lungs of the infected mice, which eventually results in the death of the animals. HKH20 inhibits activation of the contact system and protects mice with invasive S pyogenes infection from lung damage. In combination with clindamycin treatment, the peptide also significantly prolongs the survival of infected mice.
Introduction
Sepsis and septic shock are complications of bacterial infections that are, despite treatment with antibiotics and improved intensive care, associated with high mortality rates. Streptococcus pyogenes is a major human pathogen that mainly causes skin and throat infections. These infections are normally superficial and self-limiting, but they occasionally develop into the serious and life-threatening conditions streptococcal toxic shock syndrome and necrotizing fasciitis.1 The molecular mechanisms behind the pathogenesis of these critical conditions are still not fully understood. However, a growing body of evidence suggests that they are the result of an uncontrolled host inflammatory response induced by the pathogen. A systemic activation of proteolytic host cascades, such as the complement, coagulation, and contact systems, plays an important role, together with a massive release of pro-inflammatory cytokines.2
Previous work has shown that S pyogenes is able to assemble and activate the human contact system on its surface.3 The contact system, also known as the intrinsic pathway of coagulation or the kallikrein-kinin system, is involved in normal hemostasis and inflammation.4-6 It is composed of 4 components: factor XI (FXI), FXII, plasma kallikrein (PK), and high molecular weight kininogen (HK). Under physiologic conditions, these factors circulate in their inactive forms in the bloodstream or are bound to the surface of different cell types, such as endothelial cells, platelets, and polymorphonuclear neutrophils (PMNs). On activation, the contact system triggers the intrinsic pathway of coagulation via activation of FXI by FXII and evokes the release of bradykinin (BK) from the HK precursor by the action of PK. BK, a peptide consisting of 9 amino acids, is a potent pro-inflammatory mediator. Thus, BK has been shown to evoke the generation of nitric oxide and other inflammatory substances (eg, prostaglandins and leukotrienes), reduce blood pressure, and induce fever. Notably, and probably more important in respect to infectious diseases, BK also induces increased vascular permeability and capillary leakage, causing pain, edema, and hypotension.4,6 BK levels are often significantly increased in patients with sepsis and septic shock. Although the local activation of the contact system is considered to have a beneficial effect for the human host, that is, via generation of HK-derived antibacterial peptides,7 a systemic contact activation may lead to severe complications, such as kinin-induced vascular leakage and bleeding disorders.8 Several reports have described contact system activation in various animal models of infection with different pathogens.9-13 Contact activation has been seen in all animal species tested, including baboons and rats9,11 and also in mice, where the degree of activation varies between mouse strains.13 Thus, animal models of infection may be useful tools to study contact system inhibition for therapeutic purposes.
The present study investigates whether a peptide (HKH20) derived from a region of HK known to interact with bacterial surfaces could be used to block the activation of the contact system and to treat experimental S pyogenes infections. The results show that HKH20 is a potent inhibitor of the contact system. Moreover, in a mouse model of invasive S pyogenes infection, the peptide prevented pulmonary lesions. In combination with clindamycin, HKH20 significantly improved the survival rate during murine infection.
Methods
Materials
The S pyogenes strain AP1 (40/58) of serotype M1 was from the World Health Organization (WHO) Collaborating Center for Reference and Research on Streptococci (Prague, Czech Republic). Bacteria were grown in Todd-Hewitt broth (TH; Difco) at 37°C in the presence of 5% CO2. Fresh frozen plasma from healthy persons was obtained from the blood bank at Lund University Hospital (Lund, Sweden) and kept frozen at −80°C until use.
Clotting assays
All clotting times were measured using an Amelung coagulometer. Activated partial thromboplastin time (aPTT) was measured by incubating 30 μL of HKH20 or GCP28 (50 μM final concentration) diluted in sterile water, with 100 μL citrated human plasma for 1 minute followed by the addition of 100 μL aPTT reagent (aPTT Automate, Diagnostica Stago) for 60 seconds at 37°C. Clotting was initiated by the addition of 100 μL of a 25-mM CaCl2 solution. In the prothombrin time assay (PT), clotting was initiated by the addition of 100 μL Thrombomax with calcium (PT reagent; Sigma-Aldrich). For measuring the thrombin clotting time (TCT), clotting was initiated by the addition of 100 μL Accuclot thrombin time reagent (TCT reagent; Sigma-Aldrich).
Chromogenic substrate assay
Fifty-milliliter overnight cultures of S pyogenes AP1 bacteria were washed 3 times with 50 mM Tris-HCl (pH 7.5), resuspended, and diluted to a final concentration of 2 × 1010 colony-forming units (CFU)/mL in 50 mM Tris-HCl/50 μM ZnCl2 buffer. A total of 200 μL of bacteria was incubated with 60 μL of HKH20 or GCP28 (final concentration 50 or 100 μM) for 30 seconds before the addition of 200 μL human plasma. Samples were incubated for 30 minutes at 37°C with shaking. After centrifugation, pellets were washed 2 times in 50 mM Tris (pH 7.5) centrifuged, resuspended in 100 μL 50 mM Tris-HCl/50 μM ZnCl2 buffer containing 1 mM of the chromogenic substrate S-2302, and incubated for 30 minutes at 37°C. The samples were centrifuged, and the absorbance of the supernatants was measured at 405 nm. No endogenous proteolytic activity was measured when S-2303 was incubated with AP1 bacteria in the absence of plasma.
Bactericidal assay
Bacteria were grown to mid-log phase (OD ∼ 0.4 at 620 nm) in TH broth, washed, and diluted in 50 mM Tris-HCl (pH 7.5); 50 μL of bacteria (2 × 106 CFU/mL) was incubated together with HKH20 at various concentrations for 1 hour at 37°C. To quantify the bactericidal activity, serial dilutions of the incubation mixtures were plated on TH agar, incubated overnight at 37°C, and the number of CFU were determined.
Incubation of bacteria in plasma
Overnight cultures of S pyogenes AP1 bacteria were washed 3 times with 50 mM Tris-HCl (pH 7.5), resuspended, and diluted to a final concentration of 2 × 1010 CFU/mL in 50 mM Tris-HCl/50 μM ZnCl2 buffer. A total of 100 μL of bacteria was incubated with 30 μL of HKH20 or GCP28 (final concentration, 50 or 100 μM) for 30 seconds before the addition of 100 μL human plasma. Samples were incubated on a rotator at room temperature for 15 minutes unless indicated otherwise. After incubation, the bacteria were washed 2 times in 50 mM Tris (pH 7.5), centrifuged, and resuspended in 50 μL of 50 mM Tris-HCl/50 μM ZnCl2 buffer. The suspensions were allowed to stay at room temperature for 15 minutes, followed by centrifugation at 10000g. Supernatants were collected and kept at −20°C until Western blot analysis.
Electrophoresis and Western blot analysis
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Neville.16 Proteins in the supernatants from bacterium-plasma incubations were separated on gels of 10% total acrylamide with 3% bisacrylamide. Plasma samples diluted 1/100, untreated or treated with kaolin (Diagnostica Stago) for 15 minutes, served as controls. Before loading, samples were boiled in sample buffer containing 2% (wt/vol) SDS and 5% (vol/vol) beta-mercaptoethanol for 10 minutes. For Western blot analyses, proteins were transferred to nitrocellulose membranes (Immobilon, Millipore), as described previously.17 Subsequently, nitrocellulose membranes were blocked in phosphate-buffered saline (PBS)–Tween containing 5% (wt/vol) nonfat dry milk at room temperature for 30 minutes, washed 3 times with PBS-Tween for 5 minutes, and incubated with sheep antibodies against HK and its degradation products (1:3000 in the blocking buffer) at room temperature for 60 minutes. After a washing step, membranes were incubated with peroxidase-conjugated secondary donkey antibodies against goat IgG (1:3000, MP Biomedicals) at room temperature for 60 minutes. Bound secondary antibodies were detected by the chemiluminescence method.18
Animal experiments
S pyogenes AP1 bacteria grown to early logarithmic phase were washed and diluted in PBS to a concentration of 5 × 107 CFU/mL. Female BALB/c mice, 10 to 12 weeks old, were injected intraperitoneally with 100 μL of the bacterial solution, or with 100 μL of bacteria together with 100 μL HKH20 (2 mg/mL, which corresponds to 200 μg/mouse) mixed directly before injection, or with 200 μL PBS alone (control group). Alternatively, mice were treated with 50 μg aprotinin/mouse (Merck, > 5 TIU/mg protein). After 18 hours, mice were killed and spleens and lungs were removed. The spleens were kept on ice until homogenization in PBS, and the number of CFU in the spleen was quantified by plating serial dilutions of the homogenized material on blood agar plates. Lungs were further processed for microscopic analysis.
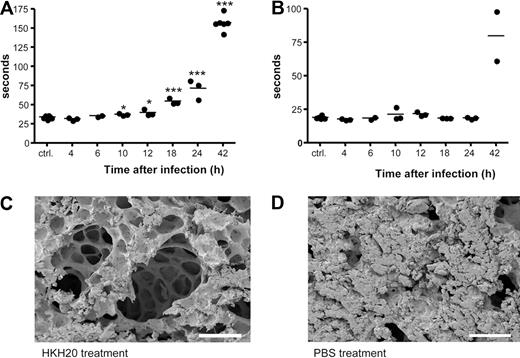
For the subcutaneous infection model, mice were anesthetized with isofluorane and injected with 2 × 107 CFU AP1 bacteria in an air pouch on the neck. Mice showed systemic signs of sickness 8 to 12 hours after infection. To measure clotting times, 8 groups of female BALB/c mice were infected; and after various time points (0, 4, 6, 10, 12, 18, 24, and 42 hours), the animals were anesthetized with isofluorane and terminal blood samples were taken. Approximately 0.5 mL of blood was drawn by cardiac puncture into polypropylene tubes containing one-tenth volume of 3.8% trisodium citrate. Plasma was separated by centrifugation, and clotting times were measured as described earlier. To determine bacterial dissemination, spleens were removed and bacterial counts were determined.
PMN depletion was induced by intraperitoneal injection of the anti–mouse Ly-6G (Gr-1) antibody (eBioscience; 100 μg/mouse) 8 hours before infection. Neutropenia was confirmed before starting the infection by manual white blood cell count and fluorescence-activated cell sorter (FACS) analysis.19 To investigate leukocyte recruitment into the peritoneal cavity, mice were killed, 5 mL PBS was injected, and after massage of the peritoneum the fluid was removed and analyzed. Statistical analysis was performed using GraphPad Prism, Version 4.00. The P value was determined using the unpaired t test (comparison of 2 groups) or the log-rank test (comparison of survival curves). All animal experiments were approved by the regional ethical committee for animal experimentation, the Malmö/Lund djurförsöksetiska nämnd, Lund District Court, Sweden (permit M209-06).
Pharmacokinetics of HKH20
HKH20 was labeled with the fluorescent dye AlexaFluor 555 (Invitrogen) according to the manufacturer's protocol. The labeled peptide or the same amount of dye (control) was injected intraperitoneally into mice. Animals were killed after 15, 30, 60, and 120 minutes. Blood samples were collected and organs (lung, spleen, liver, and kidney) removed. The fluorescence of the blood samples was measured with a Multilabel counter (PerkinElmer Life and Analytical Sciences). Tissue samples were fixed at 4°C for 24 hours in buffered 4% formalin (pH 7.4; Kebo), dehydrated, and imbedded in paraffin (Histolab Products), cut into 4-μm sections, and subjected to fluorescence microscopic analysis. After removal of paraffin, slides were mounted with ProLong Gold antifade reagent (Invitrogen).
Histochemistry and histopathologic evaluation
Mice were killed, and lungs rapidly removed and fixed at 4°C for 24 hours in buffered 4% formalin (pH 7.4; Kebo). Tissues were dehydrated and imbedded in paraffin (Histolab Products), cut into 4-μm sections, and mounted. After removal of the paraffin, tissues were stained with Mayers hematoxylin (Histolab Products) and eosin (Surgipath Medical Industries). Mouse organs were fixed in 4% paraformaldehyde (in PBS, pH 7.4) and processed for routine histopathologic evaluation. Slides were viewed with a Nikon ECLIPSE 80i microscope using a Plan APO 20×/0.75 DIC N2 lens. Images were acquired using NIs-Elements Imaging Software (Version 2.33).
Scanning electron microscopy
Lung samples from the mice were fixed as previously described.20 After fixation, samples were washed, dehydrated, critical point dried, and sputtered with palladium/gold as described earlier.21 Specimens were examined in a Jeol J-330 scanning electron microscope (JEOL) operated at an acceleration voltage of 5 kV, working distance of 10 minutes and a magnification of 300× to 2500×. Images were captured with a Satan Multiscan 791 CCD camera (Satan Inc).
Results
HKH20 inhibits the intrinsic, but not the extrinsic, pathway of coagulation
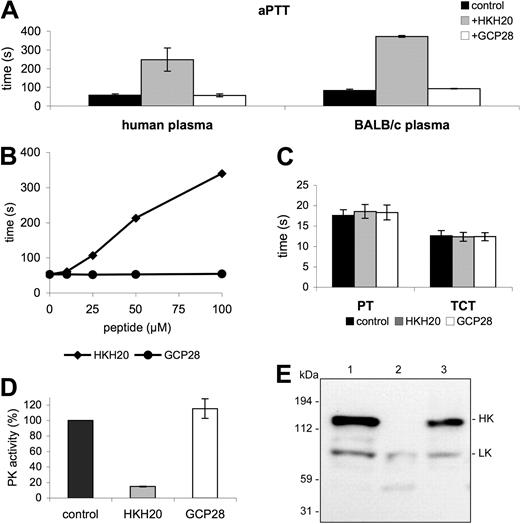
Previous studies have shown that domain 5 of HK is a potent adhesin with high affinity for negatively charged surfaces, including cellular membranes (eg, endothelial cells and neutrophils) and artificial substances, such as dextran sulfate or kaolin.14,22 Notably, the same domain has also been demonstrated to interact with bacterial surfaces,23,24 and subsequent work has led to the identification of the bacteria-binding site in domain 5. A peptide (HKH20) spanning amino acids 479 to 498 of HK was found to mimic the S pyogenes and Staphylococcus aureus binding site in HK.23,24 It has also been reported that HKH20 can displace HK from other surfaces, including cellular surfaces, such as endothelial cells, or synthetic surfaces, such as kaolin or dextran sulfate.25 Our findings that radiolabeled HKH20 binds directly to kaolin and S pyogenes (data not shown) are in line with these observations. Concerning the functional activity of HKH20, it should be mentioned that its incubation with dextran sulfate and purified PK, HK, and FXII prevents activation of the contact system.26 These properties of HKH20 suggest that it may interfere with the assembly and activation of the contact system in vitro and in vivo. Initial experiments investigated the ability of HKH20 to inhibit contact activation in plasma, and various clotting assays demonstrated that HKH20 impairs the intrinsic pathway of coagulation in normal human plasma and in mouse (BALB/c) plasma. Figure 1A shows that incubation of plasma with HKH20 led to a 4-fold increase of the aPTT compared with GCP28, a control peptide derived from domain D3 of HK. The effect of HKH20, as depicted in Figure 1B, was dose dependent. In contrast, other parts of the coagulation system as judged by the prothrombin time (PT), monitoring the extrinsic pathway of coagulation, and the TCT, measuring thrombin-induced fibrin-network formation, were not affected in human plasma (Figure 1C) or in BALB/c plasma (not shown). The results demonstrate that HKH20 exclusively targets the intrinsic pathway of coagulation.
HKH20 interferes with the intrinsic pathway of coagulation. (A) Human plasma or BALB/c mouse plasma was incubated with 50 μM HKH20, GCP28, or buffer alone (control) for 60 seconds and analyzed by the aPTT test. (B) Human plasma was incubated with increasing amounts of HKH20 or GCP28, and the aPTT was measured. (C) Normal human plasma was incubated with 50 μM HKH20, GCP28, or buffer alone (control) for 60 seconds and analyzed by the PT and the TCT tests. (D) Human plasma was incubated with kaolin in the presence of 50 μM HKH20, GCP28, or buffer alone (control) for 15 minutes. Plasma was removed by centrifugation and pelleted kaolin was washed and resuspended in substrate buffer. After 15 minutes of incubation, plasma kallikrein activity was measured in a substrate assay. Data are presented as percentage activity compared with the control; values are mean ± SD (n = 3). (E) Human plasma was incubated with buffer (lane 1), kaolin (lane 2), or kaolin and 50 μM HKH20 (lane 3) for 15 minutes. Samples were analyzed by Western blotting with antibodies identifying HK and LK.
HKH20 interferes with the intrinsic pathway of coagulation. (A) Human plasma or BALB/c mouse plasma was incubated with 50 μM HKH20, GCP28, or buffer alone (control) for 60 seconds and analyzed by the aPTT test. (B) Human plasma was incubated with increasing amounts of HKH20 or GCP28, and the aPTT was measured. (C) Normal human plasma was incubated with 50 μM HKH20, GCP28, or buffer alone (control) for 60 seconds and analyzed by the PT and the TCT tests. (D) Human plasma was incubated with kaolin in the presence of 50 μM HKH20, GCP28, or buffer alone (control) for 15 minutes. Plasma was removed by centrifugation and pelleted kaolin was washed and resuspended in substrate buffer. After 15 minutes of incubation, plasma kallikrein activity was measured in a substrate assay. Data are presented as percentage activity compared with the control; values are mean ± SD (n = 3). (E) Human plasma was incubated with buffer (lane 1), kaolin (lane 2), or kaolin and 50 μM HKH20 (lane 3) for 15 minutes. Samples were analyzed by Western blotting with antibodies identifying HK and LK.
We next investigated whether HKH20 interferes with the activation of PK at negatively charged surfaces. For these experiments, kaolin was preincubated with HKH20, GCP28, or buffer alone, followed by incubation with human plasma. After 15 minutes, unbound plasma proteins were removed by a centrifugation and washing step. PK activity at the surface of kaolin was then determined using a specific chromogenic substrate for PK (S-2302). As expected, HKH20 efficiently blocks PK activity, whereas the control peptide GCP28 has no influence on the enzymatic activity (Figure 1D). Because HK is a substrate for activated PK, Western blot analysis was used to test whether inhibition of PK activity prevents HK degradation. Kaolin was preincubated with HKH20 and then mixed with human plasma for 15 minutes. Plasma alone or plasma treated with kaolin served as negative and positive controls, respectively. Western blots of the diluted samples were stained with antibodies directed against HK and low-molecular-weight kininogen (LK). Notably, LK is a shorter splice variant of HK,27 and a polyclonal antiserum against HK will also react with LK. Figure 1E depicts intact HK at 120 kDa (Figure 1E lane 1) and processed HK after kaolin treatment (Figure 1E lane 2), which triggers the conversion of HK from a single chain to a 2-chain protein.22 When plasma was incubated with kaolin in the presence of HKH20, cleavage of HK was blocked and intact HK was detected (Figure 1E lane 3). Taken together, the results demonstrate that HKH20 inhibits kaolin-induced activation of the contact system in human plasma.
HKH20 prevents contact activation at the surface of S pyogenes bacteria
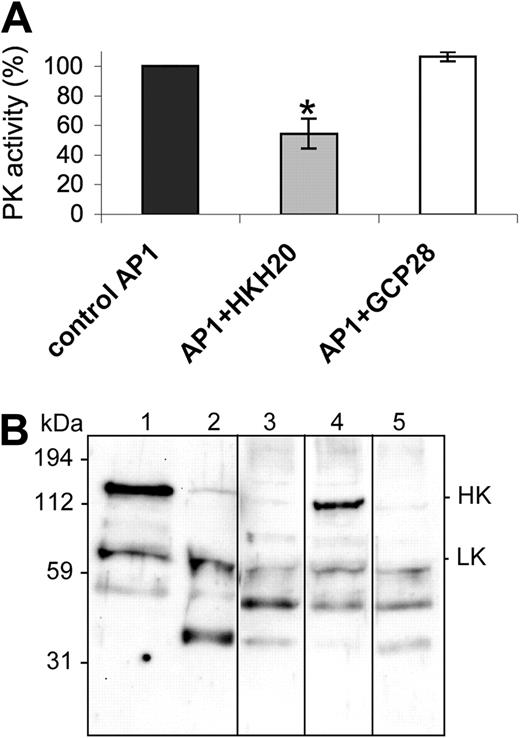
To determine whether treatment with HKH20 inhibits PK activity not only at the surface of kaolin, but also at the surface of S pyogenes, bacteria were incubated with HKH20 followed by the addition of plasma. After 30 minutes, bacteria were washed and the PK activity at the bacterial surface was determined by measuring hydrolysis of the PK substrate S-2302. The results show that treatment with HKH20 evoked a significant decrease in PK activity compared with controls incubated with buffer alone or with the GCP28 peptide (Figure 2A). Next we analyzed whether the coapplication of HKH20 prevents HK processing under these experimental conditions. The bacteria were preincubated with HKH20 for 1 minute, followed by the addition of plasma for 15 minutes. They were then washed and resuspended in buffer, followed by an additional incubation step for 15 minutes to allow the dissociation of bacteria-bound plasma proteins from the streptococcal surface. The proteins in the supernatant were analyzed by a Western blot immunostained with antibodies against HK and LK (Figure 2B). Nontreated and kaolin-treated plasma served as negative and positive controls, respectively (Figure 2B lanes 1 and 2). Western blot analysis of plasma proteins bound to and released from the streptococcal surface revealed that HK was degraded (Figure 2B lane 3). However, when bacteria were preincubated with 100 μM HKH20 before plasma was added, the degradation of HK bound to the bacteria was decreased (Figure 2B lane 4). As a control, S pyogenes was treated with peptide GCP28, which did not prevent HK cleavage (Figure 2B lane 5). The results show that the contact system is assembled and activated at the surface of S pyogenes and that HKH20 interferes with these molecular events.
HKH20 inhibits S pyogenes-induced contact activation. (A) Human plasma was incubated with S pyogenes bacteria in the presence of 50 μM HKH20, GCP28, or buffer alone (control) for 30 minutes. Plasma was removed by centrifugation, and bacteria were washed and resuspended in substrate buffer. After 30 minutes of incubation, plasma kallikrein activity was measured in a substrate assay. Data are presented as percentage activity compared with the control; values are mean plus or minus SD (n = 3). *P < .05 by t test. (B) Human plasma was incubated with S pyogenes bacteria in the presence or absence of HKH20 or GCP28 for 15 minutes. Bacteria were washed, resuspended in buffer, incubated for 15 minutes, and spun down. Supernatants and plasma (nontreated or kaolin-treated) were analyzed by SDS-PAGE and Western blotting with antibodies against HK and LK: lane 1, normal plasma; lane 2, kaolin-treated plasma; lane 3, plasma proteins absorbed and released by S pyogenes; lane 4, plasma proteins absorbed and released by S pyogenes in the presence of 100 μM HKH20; lane 5, plasma proteins absorbed and released by S pyogenes in the presence of 100 μM GCP28. Vertical lines have been inserted to indicate a repositioned gel lane.
HKH20 inhibits S pyogenes-induced contact activation. (A) Human plasma was incubated with S pyogenes bacteria in the presence of 50 μM HKH20, GCP28, or buffer alone (control) for 30 minutes. Plasma was removed by centrifugation, and bacteria were washed and resuspended in substrate buffer. After 30 minutes of incubation, plasma kallikrein activity was measured in a substrate assay. Data are presented as percentage activity compared with the control; values are mean plus or minus SD (n = 3). *P < .05 by t test. (B) Human plasma was incubated with S pyogenes bacteria in the presence or absence of HKH20 or GCP28 for 15 minutes. Bacteria were washed, resuspended in buffer, incubated for 15 minutes, and spun down. Supernatants and plasma (nontreated or kaolin-treated) were analyzed by SDS-PAGE and Western blotting with antibodies against HK and LK: lane 1, normal plasma; lane 2, kaolin-treated plasma; lane 3, plasma proteins absorbed and released by S pyogenes; lane 4, plasma proteins absorbed and released by S pyogenes in the presence of 100 μM HKH20; lane 5, plasma proteins absorbed and released by S pyogenes in the presence of 100 μM GCP28. Vertical lines have been inserted to indicate a repositioned gel lane.
HKH20 prevents lung lesions in mice infected with S pyogenes bacteria
The S pyogenes strain used in this study (AP1) belongs to one of the serotypes (M1) that is most frequently associated with severe infections.1 Unlike most strains of S pyogenes, the AP1 strain is virulent in BALB/c mice,28 which made it possible to study the effect of HKH20 in a mouse model of S pyogenes sepsis. Before testing HKH20 in the sepsis model, we performed toxicity tests to examine potential side effects of the peptide. To this end, 4 mice received intraperitoneal injections (400 μg/dose) of the peptide twice daily for a period of 6 days. Control mice were injected with PBS. Both groups of animals behaved normally, gained weight, and appeared completely healthy. Eighteen hours after the final injection, mice were killed and examined. Histopathologic analysis of heart, lung, spleen, kidney, and liver showed normal tissues with no signs of bleeding; blood cell counts and hemograms were also normal (data not shown). Clotting times of plasma samples from HKH20-treated mice were in the same range (aPTT, 36.7 ± 4.1 seconds; PT, 11.2 ± 0.5 seconds) as those from the PBS-treated mice (aPTT, 35.6 ± 4.5 seconds; PT, 11.5 ± 0.2 seconds).
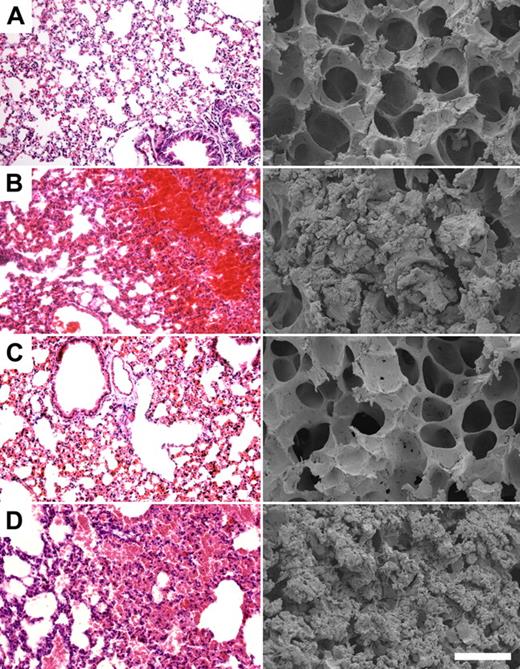
To test the effect of HKH20 in a sepsis model, mice were injected intraperitoneally with 5 × 106 CFU S pyogenes, and different groups (n = 5/group) were treated with peptides HKH20, GCP28, or with PBS. Mice injected intraperitoneally with PBS only were used as healthy controls. Eighteen hours after challenge with AP1 bacteria, all infected mice showed clear signs of sickness, such as ruffled fur and less activity. They were killed and lungs were examined by light and scanning electron microscopy. Figure 3 shows representative micrographs of lungs from noninfected mice, displaying no indication of pulmonary damage (Figure 3A), whereas mice infected with S pyogenes alone (Figure 3B) or together with peptide GCP28 (Figure 3D) had severe hemorrhage, alveolar swelling, and fibrin deposits. Such lung lesions were almost completely prevented when bacteria were injected together with HKH20 (Figure 3C). The effect of HKH20 was dose dependent; and even when the peptide was given at a lower dose (up to 100 μg/animal), a protective effect was seen (data not shown).
HKH20 prevents lung damage in BALB/c mice infected intraperitoneally with S pyogenes. Light microscopy (left) and scanning electron microscopy (right) of representative mouse lung tissue sections are shown. Mice were injected intraperitoneally with (A) 200 μL PBS, (B) 5 × 106 CFU S pyogenes, (C) 5 × 106 CFU S pyogenes and 200 μg HKH20, and (D) 5 × 106 CFU S pyogenes and 275 μg GCP28. Bars represent 250 μm (light microscopy) and 50 μm (scanning electron microscopy).
HKH20 prevents lung damage in BALB/c mice infected intraperitoneally with S pyogenes. Light microscopy (left) and scanning electron microscopy (right) of representative mouse lung tissue sections are shown. Mice were injected intraperitoneally with (A) 200 μL PBS, (B) 5 × 106 CFU S pyogenes, (C) 5 × 106 CFU S pyogenes and 200 μg HKH20, and (D) 5 × 106 CFU S pyogenes and 275 μg GCP28. Bars represent 250 μm (light microscopy) and 50 μm (scanning electron microscopy).
Analysis of the mode of action of HKH20 in vivo
Apart from blocking contact activation, HKH20 has also been reported to impair PMN recruitment25 and to be antibacterial.29 To test whether any of these properties contributes to the protective effect of the peptide, we first investigated the effect of HKH20 on neutrophil influx. Mice were infected intraperitoneally with the bacteria in the presence or absence of HKH20. Eighteen hours after infection, the animals were killed and leukocyte recruitment into the peritoneum was monitored by FACS analysis and manual counting. Figure 4 shows that the infection induced a massive invasion of leukocytes, which was not significantly changed when mice were treated with HKH20 (14.5 × 105 ± 3.2 × 105 leukocytes/mL in the untreated group vs 14.7 × 105 ± 2.7 × 105 leukocytes/mL in the HKH20-treated group). Injection of HKH20 in uninfected mice did not cause a significant influx of leukocytes (Figure 4B).
Leukocyte recruitment is not impaired by HKH20. FACS analysis of peritoneal lavage from noninfected mice injected with PBS (A) or HKH20 (B), mice injected intraperitoneally with 5 × 106 CFU S pyogenes in the absence (C) or presence (D) of 200 μg HKH20. Peritoneal lavage was analyzed 18 hours after infection. All leukocytes are CD45 positive, and neutrophil populations (red) are separated from monocyte populations (green) based on their side scatter pattern.
Leukocyte recruitment is not impaired by HKH20. FACS analysis of peritoneal lavage from noninfected mice injected with PBS (A) or HKH20 (B), mice injected intraperitoneally with 5 × 106 CFU S pyogenes in the absence (C) or presence (D) of 200 μg HKH20. Peritoneal lavage was analyzed 18 hours after infection. All leukocytes are CD45 positive, and neutrophil populations (red) are separated from monocyte populations (green) based on their side scatter pattern.
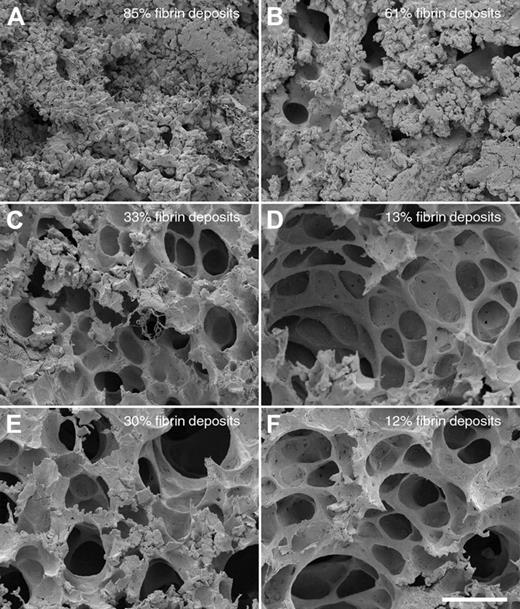
In a next series of experiments, we tested whether PMNs contribute to the lung damage in this infection model. Mice were made neutropenic by injecting a monoclonal antibody against a neutrophil surface antigen (Ag GR-1),30 which removed approximately 97% of all PMNs in the blood of the mice as determined by FACS analysis and white blood cell count (data not shown). Normal and neutropenic mice were infected intraperitoneally, in the presence or absence of HKH20 (n = 3/group) or, alternatively, challenged with aprotinin, an important inhibitor of the kallikrein/kinin system and other serine proteinases. When left untreated, neutropenic animals developed serious signs of sepsis much faster compared with normal mice, and the dissemination of bacteria to the spleen also occurred more rapidly. Ten hours after infection, all mice (untreated, treated with HKH20 or aprotinin) were killed, and lungs and spleens were removed and examined by scanning electron microscopy and bacterial colony counting. As seen before, the bacterial load in spleens of the neutropenic animals was not significantly different, regardless of the treatment (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). To quantify pulmonary lesions, lung samples from 30 different fields covering an entire lung section were made, and the percentage of the fields exhibiting hemorrhage and fibrin deposits were determined (Figure 5). Comparing normal infected and neutropenic infected mice in the absence of treatment, we found that lung lesions were reduced from 85% to 61%, respectively (Figure 5A-B), demonstrating that PMN activation and recruitment contribute to, but are not the main cause of, the damage. However, a significantly reduced damage was observed when infected mice were treated with HKH20 (Figure 5C-D) or aprotinin (Figure 5E-F) in both normal and neutropenic animals. When the lungs were analyzed by light microscopy, similar results were seen (data not shown). A potential antibacterial effect of HKH20 can be excluded because (1) there was no significant difference in the bacterial load of the spleens from normal and neutropenic animals, regardless of HKH20 treatment, and (2) the concentration of HKH20 used in these experiments was too low to be antibacterial (supplemental Figure 1B). Taken together, our data suggest that the effect of both substances (HKH20 and aprotinin) relies on the inhibition of contact system activation, rather than preventing PMN activation or recruitment.
Comparison of lung lesions in normal and neutropenic mice infected with S pyogenes. Scanning electron micrographs of representative mouse lung tissue sections are shown. Normal (A,C,E) or neutropenic (B,D,F) mice were injected intraperitoneally with (A-B) 5 × 106 CFU S pyogenes, (C-D) 5 × 106 CFU S pyogenes, and 200 μg HKH20 (E-F) 5 × 106 CFU S pyogenes and 50 μg aprotinin. Bar represents 50 μm.
Comparison of lung lesions in normal and neutropenic mice infected with S pyogenes. Scanning electron micrographs of representative mouse lung tissue sections are shown. Normal (A,C,E) or neutropenic (B,D,F) mice were injected intraperitoneally with (A-B) 5 × 106 CFU S pyogenes, (C-D) 5 × 106 CFU S pyogenes, and 200 μg HKH20 (E-F) 5 × 106 CFU S pyogenes and 50 μg aprotinin. Bar represents 50 μm.
To determine the clearance rate of HKH20, we performed a series of experiments in which fluorescently labeled HKH20 (AlexaFluor 555) was injected intraperitoneally into noninfected mice (200 μg/animal). Animals were killed after 15, 30, 60, and 120 minutes and blood and organs (lung, spleen, liver, and kidney) were removed and prepared for further examination. When measuring fluorescence in the plasma samples, we found the highest value 15 minutes after injection, whereas after 120 minutes most of the signal was gone (supplemental Figure 2). Microscopic analysis of the organs revealed an accumulation of the peptide in lung, spleen, and liver after 15 minutes, which then decreased within 2 hours after injection of the peptide, whereas it appears to accumulate in the kidney within this time (supplemental Figure 3). These findings suggest that the peptide disseminates evenly in the main organs and is cleared from plasma within 2 hours.
In vivo activation of the contact system
To examine activation of the contact system in vivo in an animal model of infection, S pyogenes bacteria were injected subcutaneously in the scruff of the neck31 at a dose of S pyogenes causing more than 95% mortality. Bacterial dissemination was followed by viable counts of spleen homogenates, and bacteria were earliest detected 10 hours after infection. Infected animals showed severe signs of disease after 18 hours, and death occurred between 24 and 64 hours after infection. To determine when contact activation occurred, plasma samples were collected from infected animals at different time points, and the clotting times of the samples were measured. Figure 6A shows that the aPTT increased in a time-dependent manner starting 10 hours after infection. In contrast, the PT was not prolonged during the first 24 hours of infection but also increased after 42 hours (Figure 6B). Based on these results and considering the clearance rate of HKH20 in plasma, we decided to administer HKH20 8 hours after infection. Electron microscopic analysis of lung biopsies from these animals revealed a significant reduction of lung lesions (Figure 6C) compared with infected mice treated with the buffer control (Figure 6D). In line with these findings, it should be mentioned that infected mice exhibited a time-dependent increase in PK activity (up to 24 hours), which was reduced in animals that received HKH20 (data not shown).
Contact activation in vivo and treatment with HKH20. Mice were injected subcutaneously in the neck with 2 × 107 CFU S pyogenes bacteria, plasma was collected at 0, 4, 6, 10, 12, 18, 24, and 42 hours after infection (n = 2-6/group), and aPTT (A) and PT (B) were measured immediately. *P < .05; ***P < .001. (C) Scanning electron microscopy of representative mouse lung tissue sections is shown. Mice were injected subcutaneously with 2 × 107 CFU S pyogenes and treated intraperitoneally 8 hours after infection with 200 μg HKH20 (C) or 100 μL PBS (D). Lungs were taken 18 hours after infection. Bar represents 100 μm.
Contact activation in vivo and treatment with HKH20. Mice were injected subcutaneously in the neck with 2 × 107 CFU S pyogenes bacteria, plasma was collected at 0, 4, 6, 10, 12, 18, 24, and 42 hours after infection (n = 2-6/group), and aPTT (A) and PT (B) were measured immediately. *P < .05; ***P < .001. (C) Scanning electron microscopy of representative mouse lung tissue sections is shown. Mice were injected subcutaneously with 2 × 107 CFU S pyogenes and treated intraperitoneally 8 hours after infection with 200 μg HKH20 (C) or 100 μL PBS (D). Lungs were taken 18 hours after infection. Bar represents 100 μm.
The effect of HKH20 treatment on survival
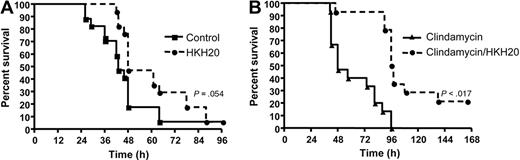
A beneficial effect of HKH20 was also demonstrated in survival studies. Subcutaneously infected mice were treated with a single dose of HKH20 8 hours after infection, and the survival was recorded. Figure 7A shows that 41% of infected mice treated with PBS died during the first 42 hours. In the HKH20-treated group, all animals were alive after 42 hours, and statistical analysis revealed that HKH20 treatment caused a significantly prolonged survival time (P = .017). Comparing the overall mortality rate of the HKH20 and PBS groups, the HKH20-treated animals showed a tendency toward prolonged survival, but this was not statistically significant (P = .054) when the experiment was terminated after 96 hours.
HKH20 improves survival in a mouse model of S pyogenes sepsis. (A) Mice were injected subcutaneously in the neck with 2 × 107 CFU S pyogenes bacteria and treated with 100 μL HKH20 (200 μg; □) or 100 μL PBS (■) intraperitoneally 8 hours after infection (n = 4 or 5/group). Mortality was recorded for a period of 5 days. The experiment was repeated 4 times, and the results from a total of 17 animals per group are shown. (B) Mice were infected subcutaneously with 2 × 107 CFU S pyogenes bacteria and treated with 10 mg/kg clindamycin (▴) or 200 μg HKH20 and 10 mg/kg clindamycin (□) in a volume of 200 μL PBS. Treatment was intraperitoneal injection at 18, 42, 48, and 72 hours after infection (n = 5/group). Mortality was recorded for a period of 7 days. The experiment was repeated 3 times, and the results from a total of 15 animals per group are shown.
HKH20 improves survival in a mouse model of S pyogenes sepsis. (A) Mice were injected subcutaneously in the neck with 2 × 107 CFU S pyogenes bacteria and treated with 100 μL HKH20 (200 μg; □) or 100 μL PBS (■) intraperitoneally 8 hours after infection (n = 4 or 5/group). Mortality was recorded for a period of 5 days. The experiment was repeated 4 times, and the results from a total of 17 animals per group are shown. (B) Mice were infected subcutaneously with 2 × 107 CFU S pyogenes bacteria and treated with 10 mg/kg clindamycin (▴) or 200 μg HKH20 and 10 mg/kg clindamycin (□) in a volume of 200 μL PBS. Treatment was intraperitoneal injection at 18, 42, 48, and 72 hours after infection (n = 5/group). Mortality was recorded for a period of 7 days. The experiment was repeated 3 times, and the results from a total of 15 animals per group are shown.
Although HKH20 administration resulted in a reversion of S pyogenes–induced lung lesions and prolonged survival times, the treatment did not significantly affect overall survival. As treatment with HKH20 did not prevent bacterial proliferation, animals might have died because of an overwhelming bacterial load. Thus, in the next series of animal experiments, the effect of HKH20 was tested in combination with clindamycin. This administration resembles the clinical situation because clindamycin is a recommended treatment for patients with invasive streptococcal infection. Initial experiments showed that S pyogenes strain AP1 is clindamycin sensitive (minimum inhibitory concentration < 0.064 mg/L, E test). Moreover, when bacteria were grown in plasma together with 10 mg/L clindamycin (156 × minimum inhibitory concentration) for 24 hours, a 97% reduction of CFUs was recorded. In contrast, HKH20 did not affect bacterial growth when added to plasma (220 μg/mL).
To test the effect of HKH20 in combination with clindamycin, mice were subcutaneously infected with S pyogenes. After 18 hours, all animals showed clear signs of advanced sepsis (bacteremia, ruffled fur, average 5% weight loss) and a significant aPTT increase (Figure 6A). This time point was then also chosen for the first intraperitoneal treatment with clindamycin (10 mg/kg) and HKH20 (200 μg/mouse). Both substances were subsequently administered intraperitoneally at 42, 48, and 72 hours after infection. In the control group, mice were injected intraperitoneally with clindamycin (10 mg/kg) only (Figure 7B). None of the animals treated with clindamycin alone survived, whereas 21% of the animals injected with clindamycin and HKH20 recovered after 168 hours. Statistical analyses revealed a median survival of 48 hours in the clindamycin-treated group versus 97 hours in the clindamycin/HKH20-treated group, which is highly significant (P < .001). These results show that HKH20, in combination with clindamycin, prolongs survival and decreases mortality in mice with severe invasive S pyogenes infection.
Discussion
Sepsis and septic shock constitute a major clinical challenge; and despite extensive efforts, only one new drug has recently been launched for the treatment of patients with severe infectious diseases. However, this drug, activated protein C (APC), is only recommended in patients at high risk of death (sepsis-induced multiple organ failure, septic shock, or sepsis-induced acute respiratory distress syndrome); and because of the anticoagulative properties of APC, the protein should not be given to patients at a risk of bleeding. Thus, there is an urgent need for novel therapies with a broader clinical indication and an improved safety profile.
Over the past 40 years, the role of the contact system in infectious diseases has attracted considerable attention.5,32,33 Several studies have shown that a massive activation of the system can trigger the generation of pathologic kinin levels and lead to a consumption of contact factors followed by impaired hemostasis. For instance, it was demonstrated as early as 1970 by Mason et al that patients with hypotensive septicemia have significantly decreased levels of contact factors,32 whereas in 1992 Pixley et al reported that low levels of FXII and HK in patients with systemic inflammatory response syndrome correlate with a fatal outcome of the disease.2 These and other findings support the notion that a systemic activation of the contact system contributes to the pathophysiology of severe and invasive infectious diseases.34
It is estimated that invasive S pyogenes infections cause more than 150000 deaths annually worldwide,35 and the prognosis is especially poor in cases with lung hemorrhage.36-38 Histologic examination of lungs recovered from patients who died of fulminant S pyogenes infection revealed severe intra-alveolar hemorrhage,38 and in these patients the aPTT was dramatically prolonged.38 Similar findings were observed in the present study when lungs and plasma samples from infected mice were analyzed. This implies that the animal model used here mimics these fulminant S pyogenes infections in humans. Our results show that the lung lesions in the infected mice are prevented when animals are treated with a contact system inhibitor. However, they also suggest that neutrophils play a role in this process because lung lesions decreased from 85% to 61%, respectively, in neutropenic mice (Figure 5A-B). This is in concordance with previous studies showing that M1 protein, a classic virulence determent of S pyogenes, is able to activate neutrophils and cause lung lesions in mice.31,39 It was also demonstrated, in a S pyogenes infection model, that pulmonary damage was prevented when neutrophil activation was blocked.31 However, in that study, the inhibitor was given before the infection became invasive (30 minutes) and the mice were killed after 6 hours, implying that the contact system was not activated at that stage. These findings may also explain the present results that HKH20 treatment of infected mice at an earlier time point (2 hours after subcutaneous infection) and before the contact system was activated had no beneficial effect.
HKH20 is derived from a region of HK responsible for binding to bacterial and eukaryotic cell surfaces.14,23 Interference with this binding was found to block the assembly and activation of the system in vitro. In previous studies, we used an irreversible contact system inhibitor to prevent severe lung lesions in a rat model of Salmonella sepsis.40 This tripeptide derivative (H-D-Pro-Phe-Arg-CMK) forms a covalent link with the catalytic pocket of PK and FXII.41,42 Because the CMK group (chloromethylketone) is toxic and the substance may also inhibit other serine proteases when given at therapeutic doses, it is an unrealistic drug candidate. HKH20, on the other hand, has a completely different mode of action. The peptide displaces HK from its binding to negatively charged surfaces but does not influence the enzymatic activity of PK and FXII. The interference with a defined protein interaction should also enhance the specificity of HKH20. Moreover, HKH20 is not cytotoxic,29 and the analysis in the present toxicity study showed that the peptide is well tolerated, also when administered to mice in doses much higher than required for a therapeutic effect. No signs of bleeding disorders in the organs were observed in the animals. In contrast to APC, which interferes with the extrinsic and the primary pathway of coagulation, HKH20 blocks the intrinsic system, which plays a secondary role in hemostasis. This and the fact that the peptide prevents lung bleedings and tissue damage, and, in combination with clindamycin, prolonged survival time and increased overall survival, could represent a novel therapeutic principle in severe infectious diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Monica Heidenholm and Maria Baumgarten for excellent technical assistance; Rita Wallén and Eric Hallberg, Cell and Organism Biology, Lund University; Lina Gefors, The Jubileum Institute, Lund University for support with electron microscopy; and Per Alm for histopathologic examination of mouse organs.
This work was supported in part by the foundations of Torsten and Ragnar Söderberg (Stockholm, Sweden), Alfred Österlund (Malmö, Sweden), Crafoord (Lund, Sweden), Greta and Johan Kock (Trelieborg, Sweden), the Blood and Defence Network (Lund, Sweden), and the Vascular Wall Programme at Lund University, the Medical Faculty, Lund University, the Swedish Research Council (Stockholm, Sweden; projects 4342, 7480 and 13413), and Hansa Medical AB (Lund, Sweden).
Authorship
Contribution: S.O. performed research, analyzed the data, and wrote the paper; O.S., A.L., and A.I.O. performed research; M.v.K.-B. contributed analytic tools; M.M. contributed analytic tools and performed research; and L.B. and H.H. designed research and wrote the paper.
Conflict-of-interest disclosure: Hansa Medical (Lund, Sweden), which in part supported this study, has filed patent applications on HKH20. S.O., M.M., L.B., and H.H. are listed as inventors, and the applications are pending. The remaining authors declare no competing financial interests.
Correspondence: Sonja Oehmcke, Department of Clinical Sciences, Division of Infection Medicine, BMC, B14, Lund University, Tornavägen 10, SE-221 84 Lund, Sweden; e-mail: Sonja.Oehmcke@med.lu.se.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal