Abstract
Dasatinib is associated with increased risk of bleeding among patients with chronic myeloid leukemia, even in the absence of thrombocytopenia, suggesting the presence of a hemostatic defect. We tested platelet aggregation in 91 patients with chronic myeloid leukemia in chronic phase either off-therapy (n = 4) or receiving dasatinib (n = 27), bosutinib (n = 32), imatinib (n = 19), or nilotinib (n = 9). All but 3 patients simultaneously receiving imatinib and warfarin had normal coagulation studies. All 4 patients off therapy had normal platelet aggregation. Impaired platelet aggregation on stimulation with arachidonic acid, epinephrine, or both was observed in 70%, 85%, and 59% of patients on dasatinib, respectively. Eighty-five percent of patients on bosutinib, 100% on nilotinib, and 33% on imatinib had normal platelet aggregation. Dasatinib 400 nM induced rapid and marked prolongation of closure time to collagen/epinephrine in normal whole blood on the PFA-100 system. In conclusion, dasatinib and, to some extent, imatinib produce abnormalities in platelet aggregometry testing.
Introduction
Dasatinib is a potent inhibitor of BCR-ABL1 and SRC family of kinases (SFK).1 Dasatinib 70 mg twice daily rendered a complete cytogenetic response in 53% of patients with chronic myeloid leukemia (CML) in chronic phase (CML-CP) after imatinib failure.2 Despite a favorable toxicity profile, dasatinib is frequently associated with toxicities, possibly as a consequence of its broad spectrum of kinase inhibition.3 In addition to inhibiting BCR-ABL1 and SFKs, dasatinib is also a potent inhibitor of KIT, PDGFR, and ephrin receptor (EphA2) tyrosine kinases.1 Inhibition of some of these kinases has been suggested to be linked to the development of some toxicities commonly observed with dasatinib such as myelosuppression and pleural effusion.4,5
Bleeding (mainly gastrointestinal and epistaxis) has been reported in 40% of patients receiving dasatinib, grade 3 or 4 in 10%.6 Some patients exhibit mucocutaneous bleeding with normal platelet counts,7 suggesting a faulty primary hemostasis. Bleeding without thrombocytopenia is less frequent with other tyrosine kinase inhibitors (TKIs). We analyzed platelet aggregation in response to several agonists in patients with CML receiving TKI therapy. We report for the first time that dasatinib and, to some extent, imatinib cause marked but reversible inhibition of platelet aggregation that may contribute to the bleeding diathesis of patients receiving this agent.
Methods
Study group
Ninety-one patients with CML-CP were evaluated: 20 receiving imatinib, 1 newly diagnosed, and 70 who, after imatinib failure, received nilotinib (n = 9), bosutinib (n = 32), or dasatinib (n = 29). Patients were followed with complete blood counts, basic coagulation studies (prothrombin time [PT], activated partial thromboplastin time [aPTT], and fibrinogen level) every 3 to 6 months. All patients provided informed consent in accordance with the Declaration of Helsinki. The conduct of this study was approved by the Institutional Review Board at M. D. Anderson Cancer Center.
Platelet aggregation test and PFA-100
Venous blood specimens were anticoagulated with 1:9 3.2% sodium citrate. Light transmittance aggregometry was performed within 4 hours in platelet-rich plasma (PRP) upon stimulation with 2 or 20 μM of adenosine diphosphate (ADP), 0.5 mg/mL of arachidonic acid (AA), 0.19 mg/mL of collagen, 0.1 mM of epinephrine, and ristocetin 0.5 mg/mL or 1.5 mg/mL with a Whole Blood Lumi-Aggregometer (BIO/DATA Corporation). The low cutoff values for weak and strong ADP, AA, collagen, epinephrine, and ristocetin at 0.5 and 1.5 mg/dL were 59%, 70%, 63%, 65%, 44%, 75%, and 1%, respectively. Platelet function analyzer 100 (PFA-100) was used as previously described.8 Briefly, epinephrine/collagen and ADP/collagen activate platelets in sodium citrate–anticoagulated whole blood from normal donors, creating aggregate formation at the aperture of the cartridge, and the closure time is recorded. In time-course experiments, dasatinib and imatinib 400 nM were incubated with blood samples from a single donor whose platelets were known to respond to epinephrine for 0, 30, 60, and 120 minutes at room temperature before PFA-100 testing.
Results and discussion
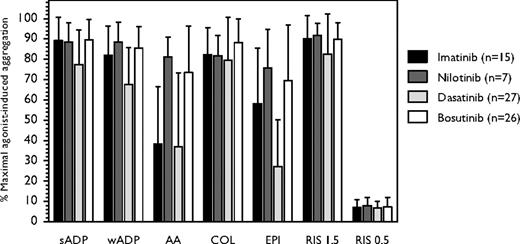
We analyzed the effect of TKIs on basic coagulation tests and platelet aggregation in 91 patients with CML-CP (Figure 1). Four patients were on no TKI therapy (2 off-dasatinib for 14 and 69 days, 1 off-imatinib for 15 days, and 1 newly diagnosed before nilotinib start), and 87 received dasatinib (n = 27), bosutinib (n = 32), imatinib (n = 19), or nilotinib (n = 9). The median age was 54 years (range, 21-80 years), white blood cell count 6.1 × 109/L (range, 2.1-58.5 × 109/L), hemoglobin 12.1 g/dL (range, 8.2-15.4 g/dL), and platelets 227 × 109/L (range, 80-746 × 109/L; > 150 × 109/L in 74 [81%] patients). Abnormal coagulation tests were observed in only 3 patients on imatinib concomitantly receiving warfarin, who exhibited prolonged PT. In the remaining 88 patients, median PT was 11.1 seconds (range, 9.8-13.1 seconds), aPTT 28.5 seconds (range, 21-33.9 seconds), and fibrinogen 470 mg/dL (range, 336-700 mg/dL), suggesting an intact secondary hemostasis. To evaluate primary hemostasis in the study cohort, platelet aggregation was measured by turbidometry in PRP upon stimulation with agonists. Twelve (13%) patients (6 bosutinib, 2 nilotinib, and 4 imatinib) were on aspirin and exhibited faulty aggregation upon AA stimulation. All 4 patients off-TKI therapy had normal platelet aggregation. At the time of aggregation testing, 46 (51%) patients had complete cytogenetic response (including 15 major and 2 complete molecular responses), 7 (8%) had partial cytogenetic response, 10 (11%) had minor cytogenetic response, 18 (19%) had complete hematologic response, and 10 (11%) had no response. None had bleeding at the time of aggregation testing.
Platelet aggregation induced by different agonists in patients with CML in chronic phase receiving therapy with different tyrosine kinase inhibitors. Dasatinib was more frequently associated with epinephrine-induced platelet aggregation defects compared with imatinib, nilotinib, and bosutinib (P < .001, one-way ANOVA) and with AA-induced platelet aggregation defects compared with nilotinib and bosutinib (P < .001, t test), but not with imatinib (P = .55, t test). Error bars represent SD.
Platelet aggregation induced by different agonists in patients with CML in chronic phase receiving therapy with different tyrosine kinase inhibitors. Dasatinib was more frequently associated with epinephrine-induced platelet aggregation defects compared with imatinib, nilotinib, and bosutinib (P < .001, one-way ANOVA) and with AA-induced platelet aggregation defects compared with nilotinib and bosutinib (P < .001, t test), but not with imatinib (P = .55, t test). Error bars represent SD.
Of the 75 patients not on aspirin, 27 (22 [81%] with at least complete cytogenetic response) were receiving dasatinib 140 mg/day (n = 1), 70 mg twice daily (n = 2), 100 mg/day (n = 10), 50 mg twice daily (n = 7), or 40 mg twice daily (n = 7). Twenty-three (85%) of them exhibited reduced epinephrine-induced platelet aggregation, 19 (70%) had reduced AA-induced platelet aggregation, and 16 (59%) exhibited impaired platelet aggregation on stimulation with both agents (Figure 2), a pattern similar to that of patients ingesting aspirin.9 These effects were independent of the dose schedule used.
Platelet aggregation in patients with CML in chronic phase receiving therapy with dasatinib or imatinib. Aggregation was recorded as increase in light transmission. Traces shown are representative of several independent tests with blood obtained from different patients treated with either dasatinib (A) or imatinib (B). Dasatinib was frequently associated with decreased response to arachidonic acid and epinephrine.
Platelet aggregation in patients with CML in chronic phase receiving therapy with dasatinib or imatinib. Aggregation was recorded as increase in light transmission. Traces shown are representative of several independent tests with blood obtained from different patients treated with either dasatinib (A) or imatinib (B). Dasatinib was frequently associated with decreased response to arachidonic acid and epinephrine.
Of the 26 patients on bosutinib (9 at 400 mg/day, 16 at 500 mg/day, and 1 at 600 mg/day) not receiving aspirin, 21 (85%) had normal platelet aggregation tests and only 4 (15%) exhibited decreased aggregation to AA (n = 2) or epinephrine (n = 2).
Of the 15 evaluable patients on imatinib (5 at 400 mg/day, 4 at 600 mg/day, 6 at 800 mg/day) not receiving aspirin, 5 had normal platelet aggregation, and 10 (66%) had impaired AA-induced platelet aggregation, including 2 (13%) with impaired epinephrine-induced aggregation. All 7 patients on nilotinib (5 at 400 mg twice daily, 2 at 200 mg twice daily) not receiving aspirin had normal platelet aggregation.
Notably, 2 patients with markedly abnormal platelet aggregation on stimulation with epinephrine (22% and 17%, respectively) while receiving dasatinib 50 mg/day and 40 mg twice daily, respectively, normalized their platelet aggregation tests upon switching to bosutinib 500 mg/day, supporting the notion that platelet dysfunction was due to dasatinib therapy and probably independent of BCR-ABL1 or SFK inhibition.
To further study the association between dasatinib and platelet aggregation dysfunction, whole blood from normal volunteers was treated with either dasatinib or imatinib 400 nM, and analyzed by PFA-100, which provides a global evaluation of primary hemostasis and platelet function. Dasatinib (but not imatinib) resulted in rapid and dramatic prolongation of the closure time in response to collagen/epinephrine but not to collagen/ADP (Table 1).
PFA-100 analysis upon addition of either dasatinib or imatinib to fresh whole blood obtained from healthy controls
| Treatment/time . | Epinephrine (normal <160 seconds) . | ADP (normal <120 seconds) . |
|---|---|---|
| No drug*, baseline | 112† | 80† |
| DMSO 0.2%, baseline | 100 | 72 |
| Dasatinib, min | ||
| 0 | 126 | 77 |
| 30 | > 300 | 84 |
| 60 | 297 | 87 |
| 120 | > 300 | 86 |
| Imatinib, min | ||
| 0 | 86 | 93 |
| 30 | 89 | 126 |
| 60 | 94 | 114 |
| 120 | 70 | 131 |
| Treatment/time . | Epinephrine (normal <160 seconds) . | ADP (normal <120 seconds) . |
|---|---|---|
| No drug*, baseline | 112† | 80† |
| DMSO 0.2%, baseline | 100 | 72 |
| Dasatinib, min | ||
| 0 | 126 | 77 |
| 30 | > 300 | 84 |
| 60 | 297 | 87 |
| 120 | > 300 | 86 |
| Imatinib, min | ||
| 0 | 86 | 93 |
| 30 | 89 | 126 |
| 60 | 94 | 114 |
| 120 | 70 | 131 |
Dasatinib (400 nM) rapidly induced prolongation of closure times from 100 seconds at baseline to > 300 seconds in response to epinephrine (but not in response to ADP) after only 30 minutes of incubation. In contrast, imatinib (400 nM) did not prolong closure times.
“No drug” indicates citrated blood only without the addition of DMSO (drug solvent), dasatinib, or imatinib.
Mean value of 2 experiments.
The mechanism whereby dasatinib induces platelet dysfunction is unknown. In vitro, AA added to normal PRP generates thromboxane A2, which induces platelet aggregation.10 Aspirin ablates this response by impairing the access of AA to the active site of platelet cyclooxygenase-1.11 The activity of dasatinib, or any of its pharmacologically active metabolites,12 on cyclooxygenase-1 is unknown. Dasatinib may also alter platelet aggregation by inhibiting key kinases in platelet homeostasis. SFKs LYN and FYN play critical roles in early platelet activation by glycoprotein VI, upstream of SYK and PLCγ2,13,14 and by integrin α2β1.15 The SFK inhibitor PD0173952 abolishes platelet aggregation and adhesion on collagen under shear.16 Interestingly, bosutinib, which like dasatinib is a multikinase inhibitor with potent activity against SFKs,3 did not cause any significant disturbance of platelet aggregation, and bleeding events have not been reported with bosutinib. The functional discrepancy observed in the ability of these compounds to perturb platelet aggregation may relate to nonoverlapping inhibitory effects of dasatinib and bosutinib on other kinases involved in platelet activation and/or aggregation. Interestingly, a subset of patients receiving imatinib exhibited platelet dysfunction, although this was manifested most prominently with AA stimulation. Interestingly nilotinib, a related agent, had no detectable inhibitory activity in this series.
In conclusion, dasatinib inhibits platelet function by impairing AA- and epinephrine-induced aggregation. This effect is not related to thrombocytopenia or the presence of clonal hematopoiesis and may account, at least in part, for the bleeding diathesis observed in dasatinib-treated patients. Albeit less frequent, impaired platelet aggregation on AA stimulation can occur with imatinib. The mechanism of this platelet dysfunction warrants further investigation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Author contribution: A.Q.C. designed research, performed research, analyzed data, and wrote the paper; X.H. performed research and analyzed data; H.K. reviewed the paper; and J.C. designed research, analyzed data, and wrote the paper.
Conflict of interest disclosure: H.K. and J.C. received research grant support from Novartis and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Jorge Cortes, MD, University of Texas M. D. Anderson Cancer Center, Department of Leukemia, Unit 428, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: jcortes@mdanderson.org.