In this issue of Blood, Yang and colleagues provide new insights into how plasma fibrinogen, fibrinogen uptake, and integrin signaling regulate P-selectin in circulating platelets.1
Membrane P-selectin expression is considered one of the most reliable markers of murine and human platelet activation. Current dogma suggests that platelets are invested with an entire pool of P-selectin derived from parental megakaryocytes. In this regard, it is generally thought that megakaryocytes synthesize P-selectin and package it into α-granules that are subsequently sorted into platelets during thrombopoiesis. Upon activation, megakaryocyte-derived P-selectin translocates to the plasma membrane of platelets where it serves as a tethering ligand that binds P-selectin glycoprotein-1 on target leukocytes, mediating adhesion and transmitting information.
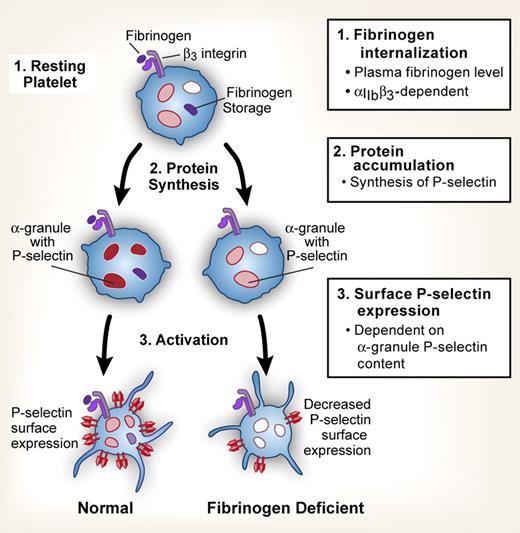
Regulation of P-selectin expression in circulating platelets. P-selectin expression is regulated on different levels: (1) internalization of fibrinogen via β3 integrins and the level of plasma fibrinogen; (2) accumulation of P-selectin by protein synthetic pathways; (3) activation-dependent P-selectin surface expression, regulated in part by the relative content of P-selectin present in α granules.
Regulation of P-selectin expression in circulating platelets. P-selectin expression is regulated on different levels: (1) internalization of fibrinogen via β3 integrins and the level of plasma fibrinogen; (2) accumulation of P-selectin by protein synthetic pathways; (3) activation-dependent P-selectin surface expression, regulated in part by the relative content of P-selectin present in α granules.
In contrast to preconceived notions, Yang et al show that platelets maintain and modulate their intracellular pool of P-selectin in the circulation. The authors demonstrate that platelets from fibrinogen (Fg) deficient mice (Fg−/−) have decreased P-selectin expression. These results are recapitulated in patients with severe hypofibrinogenemia, demonstrating their relevance to humans. The impairment is not due to excessive P-selectin shedding or the number of α-granules present in platelets. Transfusion of exogenous Fg restores the intracellular pool and activation-dependent expression of P-selectin in Fg−/− mice via engagement of the C-terminus of the Fg γ chain with β3 integrins (see figure). This suggests that the level of plasma Fg serves as a rheostat that modulates the expression of P-selectin in circulating platelets.
A primary question is how platelets dynamically alter the intracellular pool of P-selectin as they circulate in the bloodstream. To begin addressing this issue, the authors supplement Fg−/− platelets with exogenous Fg in vitro and report a marked increase in total P-selectin content. In addition, platelets from Fg−/− mice transfused with exogenous Fg increase display of surface P-selectin when activated in vitro with thrombin or a thrombin mimetic in serial studies over a 4-day period following transfusion. Together, these results suggest that Fg induces the synthesis of P-selectin by platelets. Future studies with translational inhibitors and/or incorporation of radiolabeled amino acids into newly synthesized P-selectin protein are needed to definitively prove that biosynthesis is occurring. Nevertheless, there is a precedent for this type of regulatory control in platelets. The primary receptor for Fg, integrin αIIbβ3, is synthesized by platelets2 and is also a key regulator of downstream protein synthetic responses.3 In addition, Stiegler et al recently demonstrated that copy numbers of P-selectin transcripts are tightly correlated with the amount of P-selectin protein expressed on the surface of stored human platelets,4 suggesting that translation of the mRNA may occur in the platelet as well as the megakaryocyte.5 The current study by Yang et al also supports this possibility. It is yet to be determined if internalization of fibrinogen by β3 integrins delivers an “outside-in” signal that selectively triggers translation of P-selectin mRNA as the authors suggest, or if internalized Fg in some way enhances low level constitutive protein synthesis in platelets. Both processes occur in platelets from humans and rodents.3
This fascinating study by Yang and colleagues provides further evidence that platelets can sense cues in the bloodstream, resulting in changes in the proteome of this hemostatic and inflammatory effector cell. One of these cues appears to be the level of plasma fibrinogen, which fluctuates based on one's age, immune response, or genetic makeup. Given that plasma fibrinogen alters the available pool of P-selectin stored by platelets, one has to question whether quantitative measure of surface-expressed P-selectin, by itself, is an accurate reflection of the activation state of platelets.1 Perhaps future in-depth analyses, which take into account ratios of intracellular versus surface P-selectin, the receptor density and activation state of αIIbβ3, and levels of plasma fibrinogen, are warranted.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal