Abstract
Umbilical cord blood (UCB) transplantation is potentially curative for acute leukemia. This analysis was performed to identify risk factors associated with leukemia relapse following myeloablative UCB transplantation. Acute leukemia patients (n = 177; 88 with acute lymphoblastic leukemia and 89 with acute myeloid leukemia) were treated at a single center. Patients received a UCB graft composed of either 1 (47%) or 2 (53%) partially human leukocyte antigen (HLA)–matched unit(s). Conditioning was with cyclophosphamide and total body irradiation with or without fludarabine. The incidence of relapse was 26% (95% confidence interval [CI], 19%-33%). In multivariate analysis, relapse was higher in advanced disease patients (≥ third complete remission [CR3]; relative risk [RR], 3.6; P < .01), with a trend toward less relapse in recipients of 2 UCB units (RR = 0.6; P = .07). However, relapse was lower for CR1-2 patients who received 2 UCB units (RR 0.5; P < .03). Leukemia-free survival was 40% (95% CI, 30%-51%) and 51% (95% CI, 41%-62%) for single- and double-unit recipients, respectively (P = .35). Although it is known that transplantation in CR1 and CR2 is associated with less relapse risk, this analysis reveals an enhanced graft-versus-leukemia effect in acute leukemia patients after transplantation with 2 partially HLA-matched UCB units. This trial was registered at http://clinicaltrials.gov as NCT00309842.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative therapy for patients with high-risk or relapsed acute leukemia. Myeloablative allo-HCT results in leukemia eradication not only by a direct cytotoxic effect of the intensive conditioning regimen, but also through the immune recognition of malignant cells by donor lymphocytes, referred to as the graft-versus-leukemia (GVL) reaction.1 Despite the existence of GVL, leukemia relapse following allo-HCT remains a common problem and is a major obstacle to long-term survival in transplant recipients.
Most patients who require allo-HCT lack an human leukocyte antigen (HLA)–matched sibling and identification of suitable bone marrow (BM) donors remains a challenge.2 Although BM from adult volunteer unrelated donors has been the most commonly considered alternative source of hematopoietic stem cells (HSCs), the use of unrelated umbilical cord blood (UCB) is increasing.3 Important advantages of UCB include (1) rapid donor identification and availability and (2) a low incidence of graft-versus-host disease (GVHD), despite a high degree of HLA mismatch.4-10 Because GVHD has been linked to relapse,1 there were initial concerns regarding the GVL potency of UCB. However, this has been largely resolved by single institution and registry studies, demonstrating a similar relapse risk compared with other HSC sources, including BM and peripheral blood (PB).3,9,11-13
There is growing consensus that a UCB cell dose of 2.5 × 107/kg represents the threshold of cryopreserved nucleated cells necessary for consistent engraftment.8,9,13,14 Although this cell dose is achievable with a single UCB unit for young children, it is often not possible for adult recipients. Therefore, strategies are being explored to make UCB more widely available, including ex vivo stem cell expansion,15-17 direct intra–bone marrow injection,18 enhancement of cell homing and bone marrow engraftment with CD26 blockade,19 and the use of agents that may influence the stem cell niche (ie, parathyroid hormone).20 To this end, we pioneered an approach where 2 partially HLA-matched UCB units were used to augment the progenitor cell dose in circumstances where a single unit was considered inadequate. In an earlier analysis, the addition of a second UCB unit was associated with a high incidence of engraftment, with rates comparable with those observed in children.8,21
To date, few studies have focused solely on the identification of risk factors associated with leukemia relapse following UCB transplantation. Factors previously associated with higher leukemia relapse after UCB transplantation include advanced disease status,7,8,13,22-24 high-risk cytogenetics,22 younger age (< 6 years old) and lower weight (< 21 kg),13,24 recipient cytomegalovirus (CMV) seronegativity,25 HLA match between the graft and recipient,14 and delayed recovery of antiviral immune responses.26 The goal of this analysis was to investigate risk factors for acute leukemia relapse after myeloablative conditioning and UCB transplantation at a single center with relatively homogenous treatment plans and supportive care, as well as fixed follow-up procedures and end point definitions. In addition, this is the first detailed analysis of acute leukemia patients evaluating the impact of 2 partially HLA-matched UCB units on the risk of acute leukemia relapse.
Methods
Patient eligibility
Between July 1994 and March 2008, 337 patients with acute leukemia received a UCB transplant at the University of Minnesota. Patients were excluded from this analysis if they received a nonmyeloablative conditioning (n = 113), a non–total body irradiation (TBI) conditioning regimen (n = 29), 3/6 HLA-matched units (n = 1), or coinfusion of UCB-derived natural killer cells (n = 15) or T-regulatory cells (n = 2). One hundred seventy-seven consecutive patients with acute lymphoblastic leukemia (ALL, n = 88) or acute myeloid leukemia (AML, n = 89) were eligible for this analysis. Patients who underwent transplantation in first complete remission (CR1) were considered to have high-risk leukemia if they experienced an initial induction failure (ie, ≥ 2 courses to achieve first CR), had a preceding myelodysplastic syndrome, or had evidence of high-risk cytogenetics (5q−, monosomy 7, complex cytogenetics with > 5 distinct cytogenetic abnormalities, FTL3 internal tandem duplication, t(9:22), MLL gene rearrangements (11q23)) or bilineage leukemia.27,28 For ALL patients, severe hypodiploidy or white blood cell count (WBC) higher than 50 000/mm3 at diagnosis were also considered to be high-risk features (Table 1). Other indications for transplantation in CR1, not considered to be high risk were natural killer cell leukemia (n = 1), treatment-associated leukemia without MLL gene rearrangements (n = 3), and leukemia with an associated heterozygous perforin gene mutation and hemolymphophagocytosis (n = 1). In accordance with the Declaration of Helsinki, all patients and/or guardians gave written informed consent to participate in these protocols, which were approved by the University of Minnesota institutional review board. As of July 2005, this trial was registered at http://clinicaltrials.gov (NCT00309842).
Patient demographics
| Patient characteristics . | Single unit (n = 84), no. (%) . | Double unit (n = 93), no. (%) . | P . |
|---|---|---|---|
| Year of transplant | |||
| 1994-2000 | 34 (41%) | 1 (1%) | < .01 |
| 2000-2008 | 50 (59%) | 92 (99%) | |
| Age, y | |||
| Less than 18 | 68 (81%) | 21 (23%) | < .01 |
| 18 | 16 (17%) | 72 (77%) | |
| Median (range) | 8 (0.5-52) | 24 (9-57) | |
| Median recipient weight, kg (range) | 32 (9-108) | 69 (32-149) | < .01 |
| Male recipients | 48 (57%) | 50 (54%) | .65 |
| Positive recipient CMV status | 38 (45%) | 53 (57%) | .12 |
| Median time from diagnosis to transplant, CR1 (range) | 4.1 (2.2-35.5) | 3.9 (2.2-11.3) | .47 |
| Median length of remission, CR2 (range) | 15.1 (0.8-67.3) | 21.2 (0.9-117.3) | .38 |
| Disease | |||
| ALL | 48 (57%) | 40 (43%) | .09 |
| ALL, CR1-2 | 27 (32%) | 35 (38%) | |
| ALL, CR3-rel | 13 (15%) | 5 (0.5%) | |
| AML | 34 (43%) | 53 (57%) | .36 |
| AML, CR1-2 | 27 (32%) | 44 (47%) | |
| AML, CR3-rel | 9 (11%) | 9 (9.5%) | |
| High-risk CR1 | 19/22 (86%) | 38/44 (86%) | .99 |
| Induction failure | 1 | 8 | |
| Prior MDS or 5q or 7− | 5 | 6 | |
| t(9:22) | 7 | 7 | |
| Bilineage leukemia | 1 | ||
| 5 or more cytogenetic changes | 1 | ||
| FLT3-ITD | 1 | ||
| 11q23 | 4 | 12 | |
| Hypodiploidy, for ALL | 1 | ||
| WBC more than 50 000/mm3 at diagnosis, for ALL | 1 | 2 |
| Patient characteristics . | Single unit (n = 84), no. (%) . | Double unit (n = 93), no. (%) . | P . |
|---|---|---|---|
| Year of transplant | |||
| 1994-2000 | 34 (41%) | 1 (1%) | < .01 |
| 2000-2008 | 50 (59%) | 92 (99%) | |
| Age, y | |||
| Less than 18 | 68 (81%) | 21 (23%) | < .01 |
| 18 | 16 (17%) | 72 (77%) | |
| Median (range) | 8 (0.5-52) | 24 (9-57) | |
| Median recipient weight, kg (range) | 32 (9-108) | 69 (32-149) | < .01 |
| Male recipients | 48 (57%) | 50 (54%) | .65 |
| Positive recipient CMV status | 38 (45%) | 53 (57%) | .12 |
| Median time from diagnosis to transplant, CR1 (range) | 4.1 (2.2-35.5) | 3.9 (2.2-11.3) | .47 |
| Median length of remission, CR2 (range) | 15.1 (0.8-67.3) | 21.2 (0.9-117.3) | .38 |
| Disease | |||
| ALL | 48 (57%) | 40 (43%) | .09 |
| ALL, CR1-2 | 27 (32%) | 35 (38%) | |
| ALL, CR3-rel | 13 (15%) | 5 (0.5%) | |
| AML | 34 (43%) | 53 (57%) | .36 |
| AML, CR1-2 | 27 (32%) | 44 (47%) | |
| AML, CR3-rel | 9 (11%) | 9 (9.5%) | |
| High-risk CR1 | 19/22 (86%) | 38/44 (86%) | .99 |
| Induction failure | 1 | 8 | |
| Prior MDS or 5q or 7− | 5 | 6 | |
| t(9:22) | 7 | 7 | |
| Bilineage leukemia | 1 | ||
| 5 or more cytogenetic changes | 1 | ||
| FLT3-ITD | 1 | ||
| 11q23 | 4 | 12 | |
| Hypodiploidy, for ALL | 1 | ||
| WBC more than 50 000/mm3 at diagnosis, for ALL | 1 | 2 |
UCB unit selection and characteristics
Methods of HLA typing and UCB unit selection have been detailed elsewhere.8,21 Briefly, UCB units were typed at intermediate resolution for HLA-A and -B and at allele level for HLA-DRB1. Other HLA loci were not considered in the selection algorithm. UCB units were selected if they were 2 or fewer locus HLA-mismatched with the recipient and, if 2 UCB units were used, the units had to be 2 or fewer locus HLA-mismatched with each other, as previous described.21,29 The choice to transplant 1 versus 2 UCB units was based solely on cell dose criteria. Prior to 2002, the minimum acceptable cell dose for a single unit was 1.5 × 107 nucleated cells (NCs) per kilogram, and after 2002 the minimum cell dose was 2.5 × 107/kg. After 2003, the target cell dose was 3.0 × 107 NCs/kg or higher for those with HLA-matched (ie, 6/6) units and 4.0 × 107/kg or higher for those with HLA-mismatched (ie, 4-5/6) units. If a UCB unit with the minimum cell dose was not available, the patient received a transplant of 2 partially HLA-matched UCB units.
Conditioning regimens, GVHD prophylaxis, and supportive care
All patients received a myeloablative conditioning regimen of total body irradiation (TBI) 1320 cGy (165 cGy twice daily × 4 days) and cyclophosphamide (60 mg/kg per day × 2) and identical cyclosporine immunoprophylaxis for a minimum of 180 days after UCB transplantation. However, between 1994 and 2000, the single-unit recipients received equine antithymocyte globulin (ATGAM; Pharmacia) 15 mg/kg every 12 hours on days −3 to −1 before transplantation and methylprednisolone (1 mg/kg every 12 hours from days 5 to 19) after transplantation as previously described.8 After 2000, ATGAM and methylprednisone were replaced with fludarabine (25 mg/m2 per day) on days −8 through −6 and mycophenolate mofetil (MMF, 1 g every 12 hours from day −3 to day +30),21 respectively, for all patients (single- and double-unit recipients).
Since the single-unit recipients received 2 different conditioning and acute GVHD (aGVHD) prophylaxis regimens, the outcomes of each regimen were analyzed. There were 55 patients who received the ATGAM/methylprednisolone-containing regimen and 29 patients who received the fludarabine/MMF-containing regimen. Comparing the outcomes of these 2 groups with multivariate analysis showed no differences in grade II-IV aGVHD (P = .21), treatment-related mortality (TRM; P = .06), relapse (P = .47), disease-free survival (P = .43), or overall survival (OS; P = .33). Given these similarities, the single-unit recipients were combined and considered as one group for all subsequent analysis.
All UCB units were thawed according to the methods of Rubinstein et al30 and infused after hydration and premedication with acetaminophen and diphenhydramine. UCB units were infused shortly after thawing/washing, and for patients receiving 2 units, they were infused sequentially within 30 minutes of each other. For double UCB unit transplantation, order of unit infusion was random. Granulocyte–colony-stimulating factor (5 μg/kg per day) was administered to all patients from day 1 until an absolute neutrophil count of 2.5 × 109/L or higher was achieved for 2 consecutive days. All patients received Pneumocystis jiroveci pneumonia prophylaxis with trimethoprim-sulfamethaxole or pentamidine for the first 12 months after transplantation. Viral prophylaxis included acyclovir if seropositive for herpes simplex or cytomegalovirus before transplantation. CMV surveillance was performed weekly on peripheral blood with ganciclovir treatment at the time of positive antigen or polymerase chain reaction testing.
GVHD was diagnosed clinically with histologic confirmation when possible. Staging was based upon published criteria,31 and treatment of acute GVHD (aGVHD) clinical stage II or greater was with methlyprednisolone (≥ 48 mg/m2 intravenously or oral equivalent) daily for a minimum of 2 weeks before a taper over 8 weeks.
Statistical analysis
Data on patient characteristics and transplant-related outcomes were collected by the biostatical support group at the University of Minnesota and data were analyzed as of March 29, 2009. Patient and transplant characteristics by number of donors were compared using the chi-square test for categoric data and the Wilcoxon rank-sum test for continuous data. All patients were followed longitudinally until death or last follow up. Cumulative incidence rates (and 95% confidence intervals [CIs]) were used to estimate engraftment, grade II-IV and III-IV acute GVHD (aGVHD), chronic (cGVHD), treatment-related mortality (TRM), and relapse, treating nonevent deaths as a competing risk.32 Kaplan-Meier analysis was used to estimate overall survival (OS) and leukemia-free survival (LFS).33 Univariate comparisons of all end points were completed by the log-rank test. Times of neutrophil engraftment were measured from the date of transplantation to the date of recovery (defined as the first of 3 consecutive days of absolute neutrophil count ≥ 5 × 109/L), with exclusion for early death (ie, death before day 21 without neutrophil recovery). Patients who had no engraftment by day 42 were treated as graft failures. Time to platelet engraftment was defined as a count higher than 50 × 109/L for the first 3 days without platelet transfusion support for 7 days. In recipients of 2 UCB units, the contribution of each unit to engraftment was determined from bone marrow aspiration and/or peripheral blood at days 28, 100, 365, and 720. TRM was defined as death due to causes other than the original leukemia. Relapse was defined as disease recurrence at any site. Diagnosis of relapse was supported by cytogenetic and molecular analyses when possible. Patients in continuous complete remission were censored at last follow up. Survival was defined as time from transplantation to death from any cause; leukemia-free survival (LFS) was defined as survival in patients with continuous complete remission with treatment failure occurring at the time of disease recurrence or death from any cause. A Cox proportional hazards model or the Gray-Fine method for competing hazards was used for multivariate regression.34,35 Variables included in the models were the number of donor UCB units (1 vs 2), age, weight, sex, HLA disparity, CMV serostatus, NC dose/kg, CD34+ cell dose/kg, CD3+ cell dose/kg, diagnosis and disease status at transplantation (CR1 vs CR2 vs CR3 vs > CR3 or relapse), time from diagnosis to transplantation (for relapse), conditioning regimen, GVHD prophylaxis, and development of acute GVHD (as a time-dependent covariate). All factors were tested for the proportional hazards assumption and the number of donors was included in every model.
Results
Patient characteristics, disease status, and graft characteristics
Patient characteristics are shown in Table 1. For the 177 patients, the median age was 16 years (range, 0.5-57 years) and the median weight was 58 kg (range, 9-149 kg). Forty-seven percent of patients received a transplant of 1 unit and 53%, of 2 units. Recipients of a UCB graft composed of 2 units were significantly older and had a greater median body weight compared with recipients of 1 unit (both P < .01). Most patients underwent transplantation in early stages of their disease, with 70% (n = 62) of ALL patients and 79% (n = 71) of AML patients in first or second CR. For patients in CR1, the median time from diagnosis to transplantation was not different between patients who received 1 versus 2 UCB units (4.1 months [range, 2.2-35.5 months] vs 3.9 [range, 2.2-11.3 months]; P = .47). The majority of the patients who underwent transplantation in CR1 had high-risk features regardless of whether they were received a transplant of 1 or 2 units (86% vs 86%; P = .99). For patients who underwent transplantation in CR2, the length of remission was similar between 1- and 2-unit recipients (15.1 months [range, 0.8-67.3 months] vs 21.2 months [range, 0.9-117.3 months]; P = .38). The proportion with AML versus ALL cases, their remission status (CR1-2 vs CR3 relapse), and duration of remission were similar between recipients of 1 versus 2 units.
The infused number of mononuclear cells was not different between the 2 groups. Recipients of 2 UCB units had grafts that contained a greater number of CD34+ cells (P = .04) and T cells (P < .01; Table 2). As only 1 of the 2 units engrafts long term,21,36,37 donor-recipient HLA disparity was similar between groups when considering the HLA disparity of the engrafting unit (P = .3). Specifically, a 4/6 matched unit engrafted in 53% of recipients of 2 units compared with 42% of single-unit recipients (Table 2). However, patients who received 2 units were more likely to be “exposed” to an HLA-disparate unit (P = .03; Table 2). HLA-matched (6/6) units were available for no more than 10% of patients who received a transplant of either 1 or 2 UCB units. Fifty-nine percent of single UCB recipients and 99% of double UCB recipients underwent transplantation after 2000.
Graft characteristics
| Graft characteristics . | Single unit (n = 84), median (range) . | Double unit (n = 93), median (range) . | P . |
|---|---|---|---|
| Total mononuclear cells/kg, ×107 | 3.3 (0.9-14.0) | 3.6 (1.1-6.5) | .17 |
| Smaller unit | 1.5 (0.5-2.7) | ||
| Larger unit | 2.1 (0.6-4.4) | ||
| CD34+ cell dose/kg, ×105 | 3.5 (0.4-34.8) | 4.5 (0.9-14.5) | .04 |
| Smaller unit | 1.5 (0.3-4.7) | ||
| Larger unit | 2.8 (0.5-10.6) | ||
| CD3+ cell dose/kg, ×107 | 1.0 (0.3-3.2) | 1.4 (0.5-3.1) | < .01 |
| Smaller unit | 0.5 (0.1-1.2) | ||
| Larger unit | 0.9 (0.3-2.0) | ||
| HLA match to recipient | |||
| Engrafting unit | .30 | ||
| 4/6 | 35 (42%) | 49 (53%) | |
| 5/6 | 42 (50%) | 36 (39%) | |
| 6/6 | 7 (8%) | 8 (9%) | |
| Highest amount of HLA disparity | .03 | ||
| 4/6 | 35 (42%) | 57 (65%) | |
| 5/6 | 42 (50%) | 30 (30%) | |
| 6/6 | 7 (8%) | 6 (6%) | |
| Lowest amount of HLA disparity | .72 | ||
| 4/6 | 35 (42%) | 25 (46%) | |
| 5/6 | 42 (50%) | 24 (44%) | |
| 6/6 | 7 (8%) | 5 (9%) |
| Graft characteristics . | Single unit (n = 84), median (range) . | Double unit (n = 93), median (range) . | P . |
|---|---|---|---|
| Total mononuclear cells/kg, ×107 | 3.3 (0.9-14.0) | 3.6 (1.1-6.5) | .17 |
| Smaller unit | 1.5 (0.5-2.7) | ||
| Larger unit | 2.1 (0.6-4.4) | ||
| CD34+ cell dose/kg, ×105 | 3.5 (0.4-34.8) | 4.5 (0.9-14.5) | .04 |
| Smaller unit | 1.5 (0.3-4.7) | ||
| Larger unit | 2.8 (0.5-10.6) | ||
| CD3+ cell dose/kg, ×107 | 1.0 (0.3-3.2) | 1.4 (0.5-3.1) | < .01 |
| Smaller unit | 0.5 (0.1-1.2) | ||
| Larger unit | 0.9 (0.3-2.0) | ||
| HLA match to recipient | |||
| Engrafting unit | .30 | ||
| 4/6 | 35 (42%) | 49 (53%) | |
| 5/6 | 42 (50%) | 36 (39%) | |
| 6/6 | 7 (8%) | 8 (9%) | |
| Highest amount of HLA disparity | .03 | ||
| 4/6 | 35 (42%) | 57 (65%) | |
| 5/6 | 42 (50%) | 30 (30%) | |
| 6/6 | 7 (8%) | 6 (6%) | |
| Lowest amount of HLA disparity | .72 | ||
| 4/6 | 35 (42%) | 25 (46%) | |
| 5/6 | 42 (50%) | 24 (44%) | |
| 6/6 | 7 (8%) | 5 (9%) |
Comparison of single versus double unit recipients with respect to TNC, CD34, and CD3 cell dose per kilogram. The HLA disparity between the UCB unit(s) and the host was compared for the engrafting unit, as well as the unit with the highest and lowest HLA disparity (for double recipients).
Nonrelapse events: engraftment, GVHD, and TRM
The incidence of sustained engraftment was similar for recipients of 1 versus 2 UCB units (90% vs 86%; P = .36). Time to neutrophil recovery was not different between single- and double-unit recipients (22 days [range, 9-38 days] vs 25 days [range, 8-41 days], respectively [P = .11]). Likewise, the proportion of patients achieving platelet recovery (> 5 × 1010/L) at 6 months was similar in recipients of 1 and 2 units (70% [95% CI, 58%-82%] vs 62% [95% CI, 50%-74%], respectively [P = .24]). In contrast, the incidence of grade II-IV aGVHD was significantly higher in recipients of 2 units (48% [95% CI, 37%-59%] vs 29% [95% CI, 19%-39%] P < .01), with more grade II skin GVHD, as previously reported.38 The incidence of grade III-IV aGVHD was 12% (95% CI, 4%-20%) and 25% (95% CI, 14%-36%) in recipients of single and double UCB grafts (P = .17). Similarly, chronic GVHD was marginally more frequent in recipients of 2 UCB units (single: 10% [95% CI, 4%-16%] vs double: 18% [95% CI, 10%-26%]; P = .06). In multivariate analysis, infusion of 2 UCB units was the only risk factor associated with more frequent grade II-IV aGVHD (RR = 2.1 [95% CI, 1.3-3.4]; P < .01; Table 3).
Multivariate analysis of outcomes for recipients undergoing myeloablative conditioning followed by single UCB unit or double UCB unit transplantation
| Outcome measure . | Relative risk (95% CI) . | P . |
|---|---|---|
| GVHD, grade II-IV | ||
| Number of units | ||
| One | 1.0 | < .01 |
| Two | 2.1 (1.3-3.4) | |
| Transplantation-related mortality | ||
| Number of units | ||
| One | 1.0 | |
| Two | 0.8 (0.4-1.7) | .62 |
| CD34+ | ||
| Lowest quartile, < 2.6 × 105/kg | 1.0 | |
| Upper 3 quartiles, ≥ 2.6 × 105/kg | 0.5 (0.2-0.9) | .02 |
| Age, y | ||
| Younger than 10 | 1.0 | |
| 11-17 | 4.7 (1.5-14.7) | < .01 |
| 18 or older | 5.2 (1.6-16.6) | < .01 |
| Relapse, CR1 and CR2 only | ||
| Number of units | .04 | |
| One | 1 | |
| Two | 0.5 (0.2-1.0) | |
| Disease status | ||
| CR1 | 1 | |
| CR2 | 2.2 (1.0-4.8) | .06 |
| Leukemia-free survival, all patients | ||
| Number of units | .99 | |
| One | 1 | |
| Two | 1.0 (0.6-1.6) | |
| Disease status | < .01 | |
| CR1-2 | 1 | |
| CR3 relapse | 0.5 (0.3-0.8) | |
| CD34+ cell number, × 106/kg | ||
| Less than .25 | 1 | |
| .26-.41 | 1.3 (0.8-2.3) | .32 |
| .42-.65 | 1.3 (0.7-2.3) | .44 |
| More than .65 | 1.9 (1.1-3.6) | .03 |
| Outcome measure . | Relative risk (95% CI) . | P . |
|---|---|---|
| GVHD, grade II-IV | ||
| Number of units | ||
| One | 1.0 | < .01 |
| Two | 2.1 (1.3-3.4) | |
| Transplantation-related mortality | ||
| Number of units | ||
| One | 1.0 | |
| Two | 0.8 (0.4-1.7) | .62 |
| CD34+ | ||
| Lowest quartile, < 2.6 × 105/kg | 1.0 | |
| Upper 3 quartiles, ≥ 2.6 × 105/kg | 0.5 (0.2-0.9) | .02 |
| Age, y | ||
| Younger than 10 | 1.0 | |
| 11-17 | 4.7 (1.5-14.7) | < .01 |
| 18 or older | 5.2 (1.6-16.6) | < .01 |
| Relapse, CR1 and CR2 only | ||
| Number of units | .04 | |
| One | 1 | |
| Two | 0.5 (0.2-1.0) | |
| Disease status | ||
| CR1 | 1 | |
| CR2 | 2.2 (1.0-4.8) | .06 |
| Leukemia-free survival, all patients | ||
| Number of units | .99 | |
| One | 1 | |
| Two | 1.0 (0.6-1.6) | |
| Disease status | < .01 | |
| CR1-2 | 1 | |
| CR3 relapse | 0.5 (0.3-0.8) | |
| CD34+ cell number, × 106/kg | ||
| Less than .25 | 1 | |
| .26-.41 | 1.3 (0.8-2.3) | .32 |
| .42-.65 | 1.3 (0.7-2.3) | .44 |
| More than .65 | 1.9 (1.1-3.6) | .03 |
Models included the following variables: number of donors, recipient age, recipient weight, recipient sex, donor-recipient HLA disparity, recipient CMV serostatus, UCB graft cell doses (total NC, CD34, CD3), conditioning regimen, disease, remission status at transplantation, time from diagnosis to transplantation (for relapse) and GVHD prophylaxis. Acute GVHD was included as a time-dependent variable in each of the models.
TRM at 1 year was also similar between groups (single: 26% [95% CI, 16%-36%] vs double: 29% [95% CI, 20%-38%]; P = .5). Although the number of infused units did not correlate with TRM, higher numbers of CD34+ cells/kg and younger age (< 10 years) were associated with less TRM (P < .01; Table 3). Neither sex, body weight, CMV serostatus, HLA disparity, disease type, disease remission status at transplantation, GVHD prophylaxis, nor time-dependent grade II-IV and grade III-IV aGVHD were associated with risk of TRM.
Relapse events
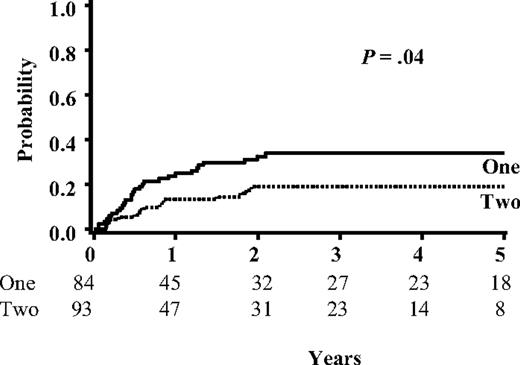
The median follow-up for single- and double-unit recipients was 5.7 years (range, 1.5-12.0 years) and 2.7 years (range, 0.5-7.0 years), respectively (P < .01). For the entire group, the median time to relapse was 209 days (range, 21-766 days), and the incidence of relapse was 26% (95% CI, 19%-33%) at 5 years. There were similar rates of relapse for patients with ALL (25% [95% CI, 15%-35%]) and AML (27% [95% CI, 17%-37%]; Table 4). Patients with advanced disease (ie, ≥ CR3 or not in remission) were more likely to relapse than those in earlier stages (CR1-2; 40% [95% CI, 23%-57%] vs 23% [95% CI, 16%-30%]; P < .01).
Incidence of relapse at 5 years
| Factor . | Relapse at 5 y (95% CI) . | P . |
|---|---|---|
| No. of units, all patients | ||
| Single | 33% (21%-45%) | 0.04 |
| Double | 19% (11%-27%) | |
| No. of units, patients in CR1-2 | ||
| Single | 31% (19%-43%) | 0.03 |
| Double | 16% (8%-24%) | |
| Disease status | ||
| ALL | 0.01 | |
| CR1 and CR2 | 21% (11%-31%) | |
| CR3 and relapsed | 40% (16%-64%) | |
| AML | ||
| CR1 and CR2 | 24% (14%-34%) | |
| CR3 and relapsed | 39% (16%-62%) | |
| Conditioning and GVHD regimen, single UCB recipients only | ||
| Cyclophosphamide/TBI methylprednisone/ATG/CSA | 33% (20%-46%) | 0.93 |
| Cyclophosphamide/fludarabine/TBI CSA/MMF | 34% (16%-52%) |
| Factor . | Relapse at 5 y (95% CI) . | P . |
|---|---|---|
| No. of units, all patients | ||
| Single | 33% (21%-45%) | 0.04 |
| Double | 19% (11%-27%) | |
| No. of units, patients in CR1-2 | ||
| Single | 31% (19%-43%) | 0.03 |
| Double | 16% (8%-24%) | |
| Disease status | ||
| ALL | 0.01 | |
| CR1 and CR2 | 21% (11%-31%) | |
| CR3 and relapsed | 40% (16%-64%) | |
| AML | ||
| CR1 and CR2 | 24% (14%-34%) | |
| CR3 and relapsed | 39% (16%-62%) | |
| Conditioning and GVHD regimen, single UCB recipients only | ||
| Cyclophosphamide/TBI methylprednisone/ATG/CSA | 33% (20%-46%) | 0.93 |
| Cyclophosphamide/fludarabine/TBI CSA/MMF | 34% (16%-52%) |
In univariate analysis there was a lower risk for relapse in double-unit transplant recipients (19% [95% CI, 11%-27%] vs 34% [95% CI, 23%-45%] at 5 years; P = .04; Figure 1 and Table 4). For patients in CR1 and CR2, relapse was significantly lower in those that received double-unit transplants compared with single-unit recipients (16% [95% CI, 8%-24%] vs 31% [95% CI, 19%-43%]; P = .03). This reduction in relapse for CR1-2 patients was observed for both for ALL and AML (Figure 2 and Table 4). Regardless of whether 1 or 2 units were used, leukemia recurrence was rare for patients in CR1 (14% [95% CI, 0%-31%] vs 13% [95% CI, 3%-23%] at 5 years; P = .70). In contrast, CR2 patients who received a transplant of a single unit showed higher relapse than double-unit recipients (40% [95% CI, 22%-58%] vs 18% [95% CI, 4%-32%] at 5 years; P = .03).
Probability of leukemia relapse for all patients based on the number of units infused. Shown are the relapse curves for all patients in this study (N = 177). The number of patients at risk for relapse is listed below each time point.
Probability of leukemia relapse for all patients based on the number of units infused. Shown are the relapse curves for all patients in this study (N = 177). The number of patients at risk for relapse is listed below each time point.
Probability of leukemia relapse based on the number of units and diagnosis for patients who underwent transplantation in CR1-2. Shown are the univariate analysis for relapse in patients who underwent transplantation in CR1-2 for ALL (n = 62) and AML (n = 71). The number of patients at risk for relapse is listed below each time point.
Probability of leukemia relapse based on the number of units and diagnosis for patients who underwent transplantation in CR1-2. Shown are the univariate analysis for relapse in patients who underwent transplantation in CR1-2 for ALL (n = 62) and AML (n = 71). The number of patients at risk for relapse is listed below each time point.
Risk of relapse was not related to modification of the treatment plan over time. All recipients of 2 UCB units received Flu in addition to cyclophosphamide/TBI and CSA/MMF. For the single-unit recipients, the conditioning and GVHD regimen differed over time (“Methods”). From 1994 to 2000, single-unit patients received a cyclophosphamide/TBI regimen containing methylprednisone and ATG. After 2000, fludarabine and MMF were substituted for these agents. Despite this, there was no difference in leukemia recurrence at 1, 2, or 5 years for single-unit recipients who underwent transplantation on these 2 regimens (P = .93; Table 4).
In multivariate analysis, after adjustment for diagnosis, disease status, and other potential risk factors, the use of 2 UCB units for early stage (CR1-2) was independently associated with a lower risk of relapse (RR = 0.5, [95% CI, 0.2-1.0]; P = .04; Table 3). Neither the conditioning regimen, GVHD prophylaxis, HLA match of the engrafting unit, graft cell dose (NCs, CD34+ and CD3+ cells/kg), nor the development of aGVHD or cGVHD was associated with relapse.
OS and LFS
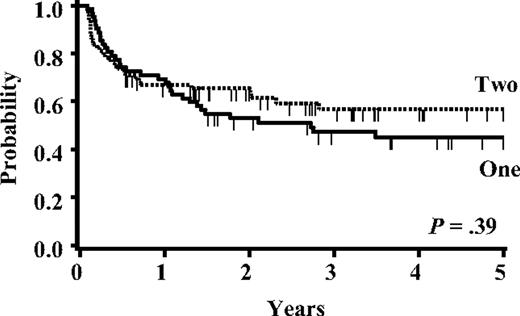
The overall survival (OS) and LFS for the whole cohort were 47% (95% CI, 39%-54%) and 46% (95% CI, 38%-53%) at 5 years, respectively. Survival was similar for patients with ALL and AML (P = .98). The 1- and 5-year LFS for single UCB recipients (55% [95% CI, 44%-65%] and 40% [95% CI, 30%-51%], respectively) was similar to double-unit recipients (58% [95% CI, 47%-67%] and 51% [95% CI, 41%-62%], respectively; P = .35). Similar results were observed for patients in CR1-2 (Figure 3). In multivariate analysis, disease status at the time of transplantation and the highest number of infused CD34+ cells were the only 2 factors independently associated with LFS (P = .01 and P = .03, respectively; Table 3).
Overall survival for CR1-2 patients based on the number of units infused.
Discussion
Until recently, the limited cell dose in a single UCB unit has been the principal obstacle to the widespread use of UCB as an HSC source, especially for patients who are older and weigh more. UCB transplantation with 2 partially HLA-matched UCB units appears to overcome this limitation.21,36,37 One striking feature of double UCB transplantation is that regardless of whether patients receive myeloablative or reduced intensity conditioning, more than 85% show rapid skewing of donor engraftment with long-term hematopoiesis derived from one UCB donor in almost all cases.21,37 Such findings may suggest an immune-based, graft-versus-graft interaction. This observation prompted us to investigate whether there were differential outcomes, particularly in the risk of leukemia relapse, after HCT using 1 versus 2 UCB units. Two risk factors for relapse were identified—disease stage at transplantation and use of 2 partially HLA-matched UCB units. To our knowledge, this is the first analysis demonstrating the potential impact of 2 partially HLA-matched UCB units on the risk of relapse in patients with acute leukemia.
In this study, the choice to receive 1 versus 2 UCB units was entirely based on the available dose of nucleated cells per kilogram of recipient body weight. Accordingly, single-unit recipients were younger and weighed less. Although a prior UCB transplant analysis identified younger recipient age and lower body weight as risk factors for leukemia recurrence,13,24 this was attributed to a disproportionate number of younger children with MLL gene rearrangement. In our analysis, the distribution of ALL and AML cases, early (CR1-2) and advanced disease stage (≥ CR3 relapse), duration of remission, and standard- and high-risk groups27,28 were similar in patients who received a transplant of 1 and 2 UCB units. When the whole cohort was analyzed using multivariate analysis, there was a nonsignificant trend toward lower relapse for double-unit recipients. Given the relatively small numbers of advanced-stage patients (CR3 relapse) and their inherent heterogeneity with respect to disease characteristics, disease burden, and prior treatment, we focused on patients who underwent transplantation in CR1-2. In univariate and multivariate analysis, CR1-2 patients had less relapse if they received a transplant of 2 UCB units. Double UCB transplantation was used only in the later time points of our study. Thus, the median follow up was shorter (2.7 years) than for single-unit recipients (5.7 years). Although it is possible that longer follow up may impact our findings, this is not likely, considering that most relapse events occur within the first 2 years following transplantation. Despite the lower rate of relapse in double-unit recipients, there was a nonsignificant increase in overall and disease-free survival, likely reflecting the limited sample size.
Considering that UCB transplant recipients receive more than 5-fold fewer lymphocytes than BM transplant recipients and that the vast majority of UCB T cells are naive, there have been concerns regarding the ability of UCB-derived lymphocytes to mediate GVL reactions. However, the incidence of leukemia recurrence following UCB transplantation is not different from that reported in recipients of BM or PB.3,9,11-13 Likewise, the rates of leukemia relapse for single-unit recipients in this study were similar to these prior UCB transplant studies.3,9,11-13 Thus, the finding that early-stage patients (CR1-2) relapse less if they receive a transplant of 2 units is highly encouraging. These results may suggest that double UCB transplant results in better disease control than either single-unit UCB transplant or more conventional sources (ie, unrelated donors BM or PB), however prospective clinical trials are required for this conclusion.
Despite the use of 1 or 2 HLA antigen–mismatched grafts in the majority of transplantations, the incidence of both grade III-IV acute and extensive chronic GVHD following UCB transplantation is low.5,8 These initial observations principally in children have also been observed in adults.9-11 Despite similarities in the degree of HLA mismatch, we have observed that recipients of 2 UCB units experience more grade II aGVHD than single-unit recipients.38 We postulated that the apparent enhanced GVL effect in recipients of 2 units might be due to the increased incidence of aGVHD. However, an association between aGVHD and relapse could not be discerned. Importantly, severe aGVHD (grade III-IV), cGVHD, and TRM did not differ between the 2 groups. Although recipients of 2 UCB units received significantly more T cells than single-unit patients, neither T-cell dose nor age was associated with grade II-IV aGVHD. We speculate that increased alloreactivity may be induced by the graft-graft interaction between the 2 UCB units, and this may be responsible for the apparent reduced risk of relapse following double UCB transplantation. However, the mechanism and effector cell population(s) remain unclear.
In summary, we compared the outcomes of patients with acute leukemia who received myeloablative conditioning and UCB transplant of either 1 or 2 partially HLA-matched UCB units. In addition to disease status at the time of transplantation, use of a UCB graft composed of 2 partially HLA-matched units is associated with significantly less acute leukemia relapse. Notably, a recent retrospective registry analysis in adults with chronic lymphoid malignancies also demonstrated lower relapse risk in those who received a transplant of 2 UCB units.39 Together, these data suggest that the use of 2 UCB units is associated with an enhanced GVL effect. However, confirmation of this observation is required before generally recommending the infusion of 2 units in all patients with acute leukemia, regardless of cell dose. Although an ongoing prospective, randomized study in children with acute leukemia will definitively address the question about the relative risk of relapse in recipients of 1 or 2 units, it is already clear that the use of 2 UCB units has markedly opened up the option of UCB transplantation to nearly all adults, with few being disqualified on the basis of an inadequate cell dose.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health National Cancer Institute P01-CA65493 (J.E.W., B.R.B., T.D., J.S.M., P.B.M.) and the Children's Cancer Research Fund (M.R.V., M.J.B., M.L.M., B.R.B., J.E.W.).
National Institutes of Health
Authorship
Contribution: M.R.V. conceived of the study and wrote the manuscript; C.G.B. participated in protocol development, reviewed the data, and wrote the manuscript; J.B., B.R.B., J.S.M., P.B.M., and D.J.W. participated in protocol development and wrote the manuscript; M.L.M. assessed GVHD and wrote the manuscript; T.D. provided statistical support; D.H.M. monitored cord blood processing and graft analysis; M.J.B. assessed leukemia risk and wrote the manuscript; and J.E.W. oversaw all aspects of this project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. Verneris, CCRB Suite 660, University of Minnesota, 425 E River Rd, Minneapolis, MN 55455; e-mail: verneris@umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal