Abstract
Viral infection or reactivation remains a major cause of morbidity and mortality after allogeneic stem cell transplantation. We now show that infusions of single cytotoxic T lymphocyte (CTL) lines (5 × 106-1.35 × 108 cells/m2) with specificity for 2 commonly detected viruses, Epstein-Barr virus (EBV) and adenovirus, can be safely administered to pediatric transplantation recipients receiving partially human leukocyte antigen–matched and haploidentical stem cell grafts (n = 13), without inducing graft-versus-host disease. The EBV-specific component of the CTLs expanded in vivo and persisted for more than 12 weeks, but the adenovirus-specific component only expanded in vivo in the presence of concomitant adenoviral infection. Nevertheless, adenovirus-specific T cells could be detected for at least 8 weeks in peripheral blood, even in CTL recipients without viral infection, provided the adenovirus-specific component of their circulating lymphocytes was first expanded by exposure to adenoviral antigens ex vivo. After infusion, none of these 13 high-risk recipients developed EBV-associated lymphoproliferative disease, while 2 of the subjects had resolution of their adenoviral disease. Hence, bispecific CTLs containing both EBV- and adenovirus-specific T cells can safely reconstitute an antigen responsive “memory” population of CTLs after human leukocyte antigen–mismatched stem cell transplantation and may provide antiviral activity. This trial was registered at www.clinicaltrials.gov as #NCT00590083.
Introduction
Latent viruses, such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV), as well as lytic agents, such as adenovirus, are responsible for significant morbidity and high mortality rates in immunocompromised persons.1,2 Conventional treatment using antiviral agents is expensive and frequently ineffective. Transfer of virus-specific T cells is an alternative means of preventing and treating these infections.3-6
We have previously shown that trivirus-specific cytotoxic T lymphocyte (CTL) lines targeting CMV, EBV, and adenovirus can be produced by genetically modifying activated monocytes and EBV-transformed B lymphoblastoid cell lines (EBV-LCLs) with a chimeric adenoviral vector expressing the immunodominant CMV-pp65 antigen.7 This approach consistently generated CTLs specific for all 3 viruses in a single culture, although characterization of their specificity revealed that the lines were dominated by CMV-reactive T cells, with a smaller fraction of EBV- and adenovirus-reactive T cells. These donor-derived, trivirus-specific CTLs could be administered safely to recipients of human leukocyte antigen (HLA)–matched related or unrelated donors (MUD) without causing graft-versus-host disease (GVHD) and with apparent activity against all 3 viruses in vivo. Strikingly, however, only the CTLs directed to EBV and CMV showed evidence of in vivo expansion and persistence.7 By contrast, adenovirus-specific CTLs were detected in the peripheral blood after infusion only in patients who also had positive adenoviral cultures.
Since pediatric stem cell recipients have a particularly high incidence of adenovirus infection,8-11 the efficacy of a CTL product with only a minor adenovirus-specific component was a concern. Therefore, we increased the frequency of adenovirus-specific T cells within our CTL lines by removing competition from the immunodominant CMV antigen and manufactured bivirus-specific CTL lines directed only to EBV and adenovirus. We could then ask whether the differences between the observed patterns of reconstitution of CMV and EBV CTLs compared with adenovirus CTLs might reflect (1) the lower frequency of adenovirus-reactive T cells contained within the trivirus CTL lines, (2) a requirement for persistent viral antigen expression as in CMV- and EBV-infected patients, or (3) different patterns of circulation and migration of T cells specific for viruses that reside in nonlymphoid (eg, adenovirus) and lymphoid tissues (eg, EBV).
This study also allowed us to determine whether bivirus-specific CTLs can be safely and effectively given to pediatric recipients of haploidentical grafts, who are at highest risk of developing viral infections after transplantation, and in whom donor-specific T cells must be able to control virus infection of recipient fibroblasts and epithelial cells that share only onehaplotype. We now describe the characterization of 20 EBV- and adenovirus-specific CTL lines and report, for the first time, the detection of potent immune responses directed against the adenovirus virion protein penton as well as to the previously described hexon.12 Furthermore, we document the safety, persistence, and antiviral activity of these CTL lines in 7 unrelated and 6 haploidentical donor stem cell transplant recipients.
Methods
Patients
The investigation was approved by the Food and Drug Administration, the Recombinant DNA Advisory Committee, the hospitals' Institutional Review Boards, and the National Marrow Donor Program's Institutional Review Board. Patient informed consent was obtained in accordance with the Declaration of Helsinki. Although the protocol was open to all patients at Texas Children's Hospital and the Methodist Hospital who were candidates for stem cell transplantation from an HLA-matched or mismatched related or unrelated donor, the subjects enrolled were those at highest risk for adenoviral infection, including children receiving a haploidentical transplant. All haploidentical transplant recipients received a CD34-selected (T cell–depleted) graft, and all patients received in vivo T-cell depletion with alemtuzumab (anti-CD52), which is associated with an increased risk of developing adenovirus infection.13 Patients received their stem cell transplant under our routine institutional conditioning and supportive care protocols.14 Clinical details are provided in Table 1. Patients who were at least 30 days after transplantation with a life expectancy more than 30 days with no severe kidney and liver disease and without GVHD of more than grade 2 were eligible to receive infusions of donor-derived bivirus-specific T cells. These CTLs were administered to 12 patients as prophylaxis treatment for adenovirus infection, whereas a 13th patient with severe adenovirus disease was treated as emergency use after FDA approval.
Patient characteristics
| UPN . | Age, y/sex . | Ethnicity . | Disease . | Donor . | Donor match (mismatched loci) . | GVHD prophylaxis . | Days after HSCT when CTL is infused . | Cell dose . | Immune suppression at time of CTL infusion . | GVHD after CTLs . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1216 | 4/F | White | AML relapse | MUD | 9/10 (HLA-A) | Alemtuzumab, FK506 and MTX | 104 | 5 × 106/m2 | None | None | Died 7 mo of relapse |
| 1217 | 3/M | White | AML CR2 | MUD | 9/10 (HLA-DQB1) | Alemtuzumab, FK506 and prednisone | 150 | 5 × 106/m2 | None | None | CCR 3.5 y |
| 1218 | 7/F | Black | NHL relapse | MUD | 9/10 (HLADQB1) | Alemtuzumab, FK506 and MTX | 134 | 5 × 106/m2 | None | None | CCR 3 y |
| 1244 | 13/F | Hispanic | MDS/PNH | HAPLO | 5/10 (HLA-A, B, Cw, DRB1, DQB1) | Alemtuzumab, and CD34 selection | 77 | 1.5 × 107/m2 | None | None | CCR 3 y |
| 1252 | 11/F | White | AML transformed from MDS | MUD | 10/10 | Alemtuzumab, CSA | 53 | 1.5 × 107/m2 | FK506 | None | CCR 3 y |
| 1265 | 9/M | White | Thalassemia | MUD | 8/10 (HLA-Cw, Cw) | Alemtuzumab, FK506 and MTX | 80 | 4.5 × 107/m2 | FK506 | None | CCR 3 y |
| 1287 | 4/M | White | CML accelerated phase | MUD | 10/10 | Alemtuzumab, FK506 and MTX | 40 | 4.5 × 107/m2 | FK506 | None | CCR 2.5 y |
| 1329 | 1/M | White | SCID | MUD | 10/10 | Alemtuzumab, CSA, prednisone | 60 | 1.35 × 108/m2 | CSA and prednisone | None | CCR 2 y |
| 1309 | 4/M | Hispanic | MDS | HAPLO | 8/10 (HLA-A, B) | Alemtuzumab, FK506, prednisone | 76 | 1.35 × 108/m2 | None | None | Alive in relapse 2.5 y |
| 1352 | 5/M | Hispanic | ALL CR1 | HAPLO | 7/10 (HLA-A, B, Cw) | Alemtuzumab, and CD34 selection | 95 | 1.35 × 108/m2 | None | None | CCR 2.5 y |
| 1358 | 12/M | Hispanic | ALL CR1 | HAPLO | 6/10 (HLA-A, Cw, DRB1, DRQ1) | Alemtuzumab, and CD34 selection | 86 | 1.35 × 108/m2 | None | None | CCR 2 y |
| 1377 | 3/F | Hispanic | ALL refractory | HAPLO | 5/10 (HLA-A, B, Cw, DRB1, DQB1) | Alemtuzumab, and CD34 selection | 77 | 1.35 × 108/m2 | None | None | Alive in relapse 2 y |
| 1406 | 10/M | Black | Secondary AML | HAPLO | 7/10 (HLA-A, B, Cw) | Alemtuzumab, and CD34 selection | 70 | 1.35 × 108/m2 | None | None | Died 10 mo of relapse |
| UPN . | Age, y/sex . | Ethnicity . | Disease . | Donor . | Donor match (mismatched loci) . | GVHD prophylaxis . | Days after HSCT when CTL is infused . | Cell dose . | Immune suppression at time of CTL infusion . | GVHD after CTLs . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1216 | 4/F | White | AML relapse | MUD | 9/10 (HLA-A) | Alemtuzumab, FK506 and MTX | 104 | 5 × 106/m2 | None | None | Died 7 mo of relapse |
| 1217 | 3/M | White | AML CR2 | MUD | 9/10 (HLA-DQB1) | Alemtuzumab, FK506 and prednisone | 150 | 5 × 106/m2 | None | None | CCR 3.5 y |
| 1218 | 7/F | Black | NHL relapse | MUD | 9/10 (HLADQB1) | Alemtuzumab, FK506 and MTX | 134 | 5 × 106/m2 | None | None | CCR 3 y |
| 1244 | 13/F | Hispanic | MDS/PNH | HAPLO | 5/10 (HLA-A, B, Cw, DRB1, DQB1) | Alemtuzumab, and CD34 selection | 77 | 1.5 × 107/m2 | None | None | CCR 3 y |
| 1252 | 11/F | White | AML transformed from MDS | MUD | 10/10 | Alemtuzumab, CSA | 53 | 1.5 × 107/m2 | FK506 | None | CCR 3 y |
| 1265 | 9/M | White | Thalassemia | MUD | 8/10 (HLA-Cw, Cw) | Alemtuzumab, FK506 and MTX | 80 | 4.5 × 107/m2 | FK506 | None | CCR 3 y |
| 1287 | 4/M | White | CML accelerated phase | MUD | 10/10 | Alemtuzumab, FK506 and MTX | 40 | 4.5 × 107/m2 | FK506 | None | CCR 2.5 y |
| 1329 | 1/M | White | SCID | MUD | 10/10 | Alemtuzumab, CSA, prednisone | 60 | 1.35 × 108/m2 | CSA and prednisone | None | CCR 2 y |
| 1309 | 4/M | Hispanic | MDS | HAPLO | 8/10 (HLA-A, B) | Alemtuzumab, FK506, prednisone | 76 | 1.35 × 108/m2 | None | None | Alive in relapse 2.5 y |
| 1352 | 5/M | Hispanic | ALL CR1 | HAPLO | 7/10 (HLA-A, B, Cw) | Alemtuzumab, and CD34 selection | 95 | 1.35 × 108/m2 | None | None | CCR 2.5 y |
| 1358 | 12/M | Hispanic | ALL CR1 | HAPLO | 6/10 (HLA-A, Cw, DRB1, DRQ1) | Alemtuzumab, and CD34 selection | 86 | 1.35 × 108/m2 | None | None | CCR 2 y |
| 1377 | 3/F | Hispanic | ALL refractory | HAPLO | 5/10 (HLA-A, B, Cw, DRB1, DQB1) | Alemtuzumab, and CD34 selection | 77 | 1.35 × 108/m2 | None | None | Alive in relapse 2 y |
| 1406 | 10/M | Black | Secondary AML | HAPLO | 7/10 (HLA-A, B, Cw) | Alemtuzumab, and CD34 selection | 70 | 1.35 × 108/m2 | None | None | Died 10 mo of relapse |
UPN indicates unique patient number; F, female; M, male; GVHD, graft-versus-host disease; CTL, cytotoxic T lymphocyte; HSCT, hematopoieitic stem cell transplantation; AML, acute myeloid leukemia; MUD, matched unrelated donor; HLA, human leukocyte antigen; alemtuzumab, anti-CD52 monoclonal antibody; FK506, tacrolimus; MTX, methotrexate; CCR, continuous complete remission; NHL, non-Hodgkin lymphoma; MDS, myelodysplastic syndrome; PNH, paroxysmal nocturnal hemoglobinuria; HAPLO, mismatched related haploidentical donor; CML, chronic myeloid leukemia; SCID, severe combined immunodeficiency; ALL, acute lymphoblastic leukemia; and CR, complete remission.
Vectors
The Ad5f35null vector for clinical use was generated by the CAGT vector production facility with funding from the National Gene Vector Laboratory using previously described methodology.15 For the generation of Ad5f35null vector, the pShuttle vector (#K1650-1, Adeno-X Expression System; Clontech) was subcloned into the pAd5f35 backbone, in which the Ad5 fiber was modified with parts of the Ad35 fiber to alter tropism. The complete genome-pAd5f35null was then transfected into 293 cells (CRL-1573; ATCC) for homologous recombination. The titer of Ad5f35null vector was 4.69 × 1011 virus particles (vp)/mL and 4.67 × 109 infectious units/mL, determined on 293 cells. The clinical grade vector was confirmed to be negative for replication-competent adenovirus by plaquing on HeLa cells and replication competent adenovirus was not generated during the T-cell expansion.
Generation of EBV-transformed B cell lines
After consent, we obtained 40 to 100 mL of peripheral blood from the stem cell donors. Then 5 × 106 peripheral blood mononuclear cells (PBMCs) were infected with concentrated EBV supernatant from our B95-8 working cell bank in the presence of cyclosporin A (Sandoz) to establish an EBV-LCL.16 EBV-LCLs were grown for at least 2 weeks in acyclovir before being used as antigen-presenting cells (APCs), to prevent the release of infectious virus. The remaining PBMCs were cryopreserved for CTL generation.
Generation of bivirus-specific CTL cultures
CTL lines were produced from adenovirus and EBV-seropositive donors. To generate bivirus-specific CTLs, up to 5 × 107 donor PBMCs were infected with Ad5f35null vector at a multiplicity of infection (MOI) of 200 vp/cell after overnight adherence in a 24-well plate.17 From day 10, the responder cells were restimulated weekly with irradiated autologous LCL transduced at an MOI of 500 vp with the same Ad5f35null vector at a responder/stimulator ratio of 4:1 as previously described.7,18 A total of 50 to 100 U/mL recombinant human interleukin-2 (R&D Systems) was added twice weekly from day 14. CTL lines were cultured in complete medium (RPMI 1640 medium; HyClone Laboratories) containing 10% fetal calf serum (HyClone Laboratories), and 2 mM l-glutamine (Biowhittaker) supplemented with 45% Clicks medium (EHAA, Irvine Scientific). After 3 or 4 stimulations (when adequate cell numbers were reached), the CTLs were cryopreserved. Release criteria for CTL included viability more than 70%, negative culture for bacteria and fungi after 7 days, endotoxin testing less than 5 EU/mL, negative result for Mycoplasma, less than 10% killing of recipient lymphoblasts at a 20:1 ratio, less than 2% CD19+ B cells, less than 2% CD14+ monocytes, and HLA identity. Twenty CTL lines were produced and characterized, 13 of which were infused. Reasons for noninfusion of the remaining 7 lines were patient ineligibility because of GVHD, severe infection, early relapse, or failure to engraft.
Immunophenotyping
CTL lines were stained with CD3, CD4, CD8, CD14, CD16, CD56, CD62L, CD45RA, CCR7, T-cell receptor-αβ, T-cell receptor-γδ, and CD19 (BD Biosciences). For each sample, 10 000 cells were analyzed by FACSCalibur with the CellQuest Software (BD Biosciences).
Determination of antigen specificity
To determine the antigen specificity of the CTL lines, 2 immunologic assays were used: cytotoxicity by chromium (Cr) release, and interferon-γ (IFN-γ) release by enzyme-linked immunospot (ELIspot) assay. The ELIspot assay provided both functional and quantitative data on the antigen-specificity of the CTL lines. Furthermore, using adenovirus pepmixes spanning hexon and penton (JPT Peptide Technologies GmbH), we were able to differentiate EBV from the adenovirus-specific activity.
Cytotoxicity assay
The cytotoxic specificity of each CTL line was analyzed in a standard 4-hour 51Cr release assay using effector/target (E/T) ratios of 40:1, 20:1, 10:1, and 5:1. EBV specificity was determined using the autologous LCLs, and Ad5f35null-transduced LCLs tested both EBV- and adenovirus-specific T cells. Patient-derived phytohemagglutinin (PHA) blasts acted as a specificity control and to test for GVHD reactivity.
ELIspot assay
ELIspot analysis was used to quantitate the frequency and function of T cells that secreted IFN-γ in response to adenovirus and EBV antigens and epitopes. ELIspots were performed on the donor CTL lines and on patient PBMCs before and after CTL infusion. To avoid interassay variability, patient samples were cryopreserved and batched for ELIspot analysis after at least 3 months of follow-up. As a positive control, PBMCs were stimulated with Staphylococcal enterotoxin B (1 μg/mL; Sigma-Aldrich). Stimulator cells were irradiated LCL (40 Gy), irradiated donor PBMCs (40 Gy), either alone and or transduced with the Ad5f35null vector (MOI of 200 vp/cell). The transduction was performed 24 to 48 hours before ELIspot setup, and after transduction the PBMCs were washed and resuspended at 106/mL in X-VIVO 15 (BioWhittaker Lonza). Hexon and penton pepmixes, diluted to 1 μg/mL, were also used as a stimulus. Responder patient PBMCs were thawed 24 hours before assay setup and cultured in CTL media (without interleukin-2), then harvested and resuspended at 2 × 106/mL. Ninety-six well filtration plates (MultiScreen, #MAHAS4510, Millipore) were coated with 10 μg/mL anti–IFN-γ antibody (Catcher-mAB91-DIK, Mabtech) overnight at 4°C, then washed and blocked with ELIspot medium for 1 hour at 37°C. Responder and stimulator cells were incubated for 20 hours, and the plates washed and incubated with the secondary biotin conjugated anti–IFN-γ monoclonal antibody (Detector-mAB, 7-B6-1-Biotin; Mabtech) followed by incubation with avidin-biotinylated horseradish peroxidase complex (Vectastain Elite ABC Kit, Standard, #PK6100; Vector Laboratories) and then developed with AEC substrate (Sigma-Aldrich).12,19,20 Plates were sent for evaluation to Zellnet Consulting, and we plotted SFCs versus input cell numbers.
Detection of EBV and adenovirus-DNA in PBMCs by quantitative real-time PCR
To quantitate EBV load in patient blood, DNA was isolated from 3 to 5 × 106 PBMCs using an anion exchange column (QIAGEN). A total of 500 ng of DNA was analyzed by quantitative polymerase chain reaction (PCR) as previously described.21 Adenovirus DNA was quantitated by Viracor or at Texas Children's Hospital using previously published protocols.22-24 Briefly, QIAGEN blood and stool mini-kits were used to extract DNA from blood and stool samples, respectively, per the manufacturer's instructions, except that elution of DNA was carried out in 100 μL of buffer to concentrate DNA. Quantitative PCR assay was performed on these samples to detect the presence of adenoviral DNA using the methodology previously published by Heim et al,24 which detects all 51 serotypes of adenovirus known to infect humans. Subgrouping analyses of positive DNA samples used the subgroup-specific quantitative PCR assay published by Lion et al.25 To confirm that the chimeric Ad5f35null vector was not transferred to the infused patients, we used real-time PCR amplification of pre- and postinfusion blood samples with specific primers spanning the chimeric region of the Ad5f35 vector.
Statistical analysis
All in vitro data were presented graphically and summarized by mean plus or minus 1 SEM or median with range. Smoothing splines were fitted to describe the trend over time for T-cell frequency data. Differences between means were analyzed by Student t tests after appropriate log transformation, and differences between medians were analyzed by the nonparametric Wilcoxon rank-sum test. P value of .05 or less was considered statistically significant.
Results
Bivirus-specific CTL lines generated for haploidentical and MUD recipients were phenotypically similar
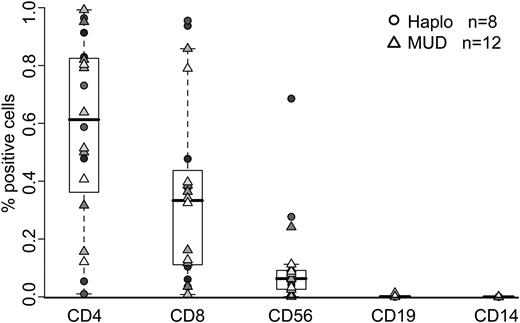
All bivirus-specific CTL lines were generated from normal donors who were donating marrow or peripheral blood stem cells to HLA-matched or mismatched unrelated recipients (n = 12) or to HLA haploidentical recipients (n = 8) (Table 1). The haploidentical donors were matched at 5 of 10 antigens (n = 3), 6 of 10 antigens (n = 1), 7 of 10 antigens (n = 3), or 8 of 10 antigens (n = 1). These 20 CTL lines were made from peripheral blood using monocytes and autologous EBV-LCLs, both of which we transduced with the Ad5f35null vector to generate APCs. Figure 1 shows the phenotype of all 20 polyclonal clinical-grade CTL lines. There was a predominance of CD4+ T cells (median, 61.2%; range, 1%-99.2%), but all CTL lines also contained the CD8+ subset (median, 33.3%; range, 0.8%-95.5%). Flow cytometric analysis of memory markers revealed mixed populations of CD45RA− CD62L− T cells (55.16% ± 32.03%) and CD45RA− CD62L+ T cells (34.48% ± 30.87%). There was no evidence of genetically modified EBV-LCL (CD19+) or monocytes (CD14+). Because all the CTL lines were made from healthy subjects, we anticipated no differences between CTLs intended for HLA-matched (round symbols) and haploidentical recipients (triangles); and as Figure 1 shows, such differences were indeed absent.
Immunophenotype of bivirus-specific CTL lines generated for clinical use. Reactivity of CTL lines (n = 20) with antibodies against the T-cell surface antigens CD3, CD4, CD8, and CD56, the monocyte surface antigen CD14, and the B-cell surface antigen CD19. Lines made from haploidentical donors are represented by circles (n = 8), and those made from matched unrelated donors are represented by triangles (n = 12).
Immunophenotype of bivirus-specific CTL lines generated for clinical use. Reactivity of CTL lines (n = 20) with antibodies against the T-cell surface antigens CD3, CD4, CD8, and CD56, the monocyte surface antigen CD14, and the B-cell surface antigen CD19. Lines made from haploidentical donors are represented by circles (n = 8), and those made from matched unrelated donors are represented by triangles (n = 12).
CTL lines are specific for EBV and adenovirus antigens
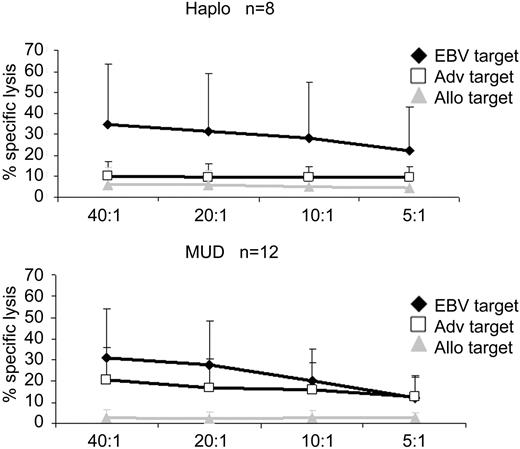
The specificity and functionality of the lines were assessed using Cr release cytotoxicity assays and IFN-γ ELIspot. All 20 donor CTL lines had cytolytic activity against EBV-LCL (mean, 35.8%; SE, 9.9% for HLA haploidentical lines; mean, 24.8%; SE, 5.1% for lines from unrelated HLA matched donors at an E/T ratio 20:1, P = .30, t test). Adenovirus-transduced donor LCLs were also recognized and killed. Adenovirus-specific killing was calculated as the difference between EBV-LCL and Ad5f35-transduced EBV-LCL killing (mean, 8.9%; SE, 2.3% for HLA haploidentical lines and mean, 17.9%; SE, 3.9% for lines from unrelated donors at an E/T ratio 20:1, P = .10, t test). The percentage specific lysis in the 4-hour Cr51 release assay was lower for adenovirus than for EBV targets, probably reflecting the predominance of CD4+ adenovirus-specific T cells, whose primary mechanism of lysis is the perforin/granzyme pathway, which requires E/T coincubation of more than 4 hours to detect specific killing. Importantly, because there have been several reports describing the cross-reactive recognition of alloantigens by virus-specific T cells, we used recipient-derived PHA blasts as a measure of the alloreactive potential of the lines and showed that these were not killed (Figure 2).
Virus-specific activity of CTL as determined by Cr release assay. 51Cr release at 4 hours after coincubation of CTL lines with autologous nontransduced EBV-LCL (EBV target), autologous EBV-LCL transduced with Ad5f35null vector, or recipient PHA blasts (allogeneic target). Lines made from haploidentical donors are shown in the top panel (n = 8) and those made from matched unrelated donors are shown in the bottom panel (n = 12). The data are mean percentage lysis (± SEM) of targets by all 20 CTL lines at E/T ratios of 40:1, 20:1, 10:1, and 5:1.
Virus-specific activity of CTL as determined by Cr release assay. 51Cr release at 4 hours after coincubation of CTL lines with autologous nontransduced EBV-LCL (EBV target), autologous EBV-LCL transduced with Ad5f35null vector, or recipient PHA blasts (allogeneic target). Lines made from haploidentical donors are shown in the top panel (n = 8) and those made from matched unrelated donors are shown in the bottom panel (n = 12). The data are mean percentage lysis (± SEM) of targets by all 20 CTL lines at E/T ratios of 40:1, 20:1, 10:1, and 5:1.
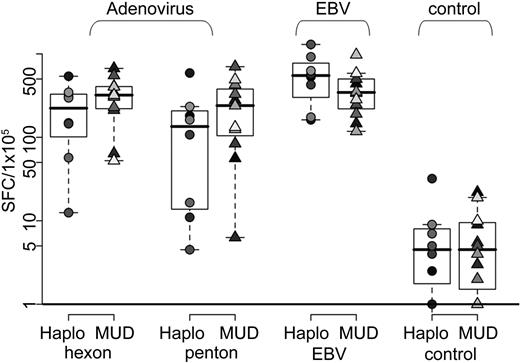
To quantify the frequency of antigen-reactive cells, we measured IFN-γ release after stimulating the T cells with pepmixes spanning the immunodominant adenovirus antigens (hexon and penton) or with irradiated autologous EBV-LCL (Figure 3). We obtained specific responses to both viruses from all 20 CTL lines. There was no significant difference in the magnitude of the responses detected between the lines given to haploidentical or unrelated donors. Results are shown in Table 2. Unstimulated CTL (data not shown) and CTL stimulated with an irrelevant pepmix served as negative controls and did not induce IFN-γ production as shown in Figure 3. Importantly, the frequency of adenovirus hexon-specific T cells in our bivirus CTL was significantly higher than in our trivirus CTL study7 (median, 86 SFC/105 CTL; range, 46-350 SFC/105 CTLs) for trivirus-specific CTLs versus median, 308 SFC/105 (range, 12-670 SFC/105 CTLs) in bivirus-specific CTL lines, respectively (P = .006). Penton-specific reactivity was not analyzed in the trivirus study.
Virus-specific activity of CTL as determined by IFN-γ ELIspot assay. T-cell specificity and cytokine production were assessed using the IFN-γ ELIspot assay by direct stimulation with hexon pepmix (adenovirus), penton pepmix (adenovirus), irradiated EBV-LCL at an E/T ratio of 1:1 (EBV), and an irrelevant pepmix (control). Results are presented as SFCs/105 CTLs. Lines made from haploidentical donors are represented by circles (n = 8), and those made from matched unrelated donors are represented by triangles (n = 12).
Virus-specific activity of CTL as determined by IFN-γ ELIspot assay. T-cell specificity and cytokine production were assessed using the IFN-γ ELIspot assay by direct stimulation with hexon pepmix (adenovirus), penton pepmix (adenovirus), irradiated EBV-LCL at an E/T ratio of 1:1 (EBV), and an irrelevant pepmix (control). Results are presented as SFCs/105 CTLs. Lines made from haploidentical donors are represented by circles (n = 8), and those made from matched unrelated donors are represented by triangles (n = 12).
CTL line ELIspot results
| . | HAPLO, SFC/1 × 105 ± SE . | MUD, SFC/1 × 105 ± SE . | P (t test) . |
|---|---|---|---|
| Adenovirus-hexon | 232.3 ± 61.4 | 323.3 ± 51.4 | .27 |
| Adenovirus-penton | 163.3 ± 68.2 | 261.4 ± 64.3 | .32 |
| EBV | 588.9 ± 133.5 | 390.0 ± 68.4 | .16 |
| . | HAPLO, SFC/1 × 105 ± SE . | MUD, SFC/1 × 105 ± SE . | P (t test) . |
|---|---|---|---|
| Adenovirus-hexon | 232.3 ± 61.4 | 323.3 ± 51.4 | .27 |
| Adenovirus-penton | 163.3 ± 68.2 | 261.4 ± 64.3 | .32 |
| EBV | 588.9 ± 133.5 | 390.0 ± 68.4 | .16 |
CTL indicates cytotoxic T lymphocyte; HAPLO, mismatched related haploidentical donor; MUD, matched unrelated donor; and EBV, Epstein-Barr virus.
Bivirus-specific CTL did not cause significant toxicity in vivo
The safety of the bispecific donor CTLs was assessed in 13 patients (6 haploidentical patients matching at 5 of 10 antigens [n = 2], 6 of 10 antigens [n = 1], 7 of 10 antigens [n = 2], or 8 of 10 antigens [n = 1]; and 7 unrelated recipients matching at 8 of 10 antigens [n = 1], 9 of 10 antigens [n = 3], and 10 of 10 antigens [n = 3]) who had undergone hematopoietic stem cell transplantation (HSCT) to treat leukemia or congenital diseases (Table 1). Twelve patients were treated on-study and one received a compassionate dose of CTLs. Each patient received from 5 × 106 to 1.35 × 108 cells/m2 at 40 to 150 days after HSCT (median, 77 days; range, 40-150 days). None of the 13 patients developed toxicities related to CTL therapy during the 3-month postinfusion safety monitoring period (Table 1). Although all persons were high-risk recipients of alternative donor transplants, no subject developed de novo GVHD after CTL therapy.
Outcome of donor-derived bivirus-specific CTL infusions
The in vivo expansion and antiviral activity of the bispecific CTL were assessed in 12 of the 13 patients. There were insufficient cells isolated from patient 9 resulting from small volumes of blood collected, so this patient was excluded from analysis.
EBV specificity
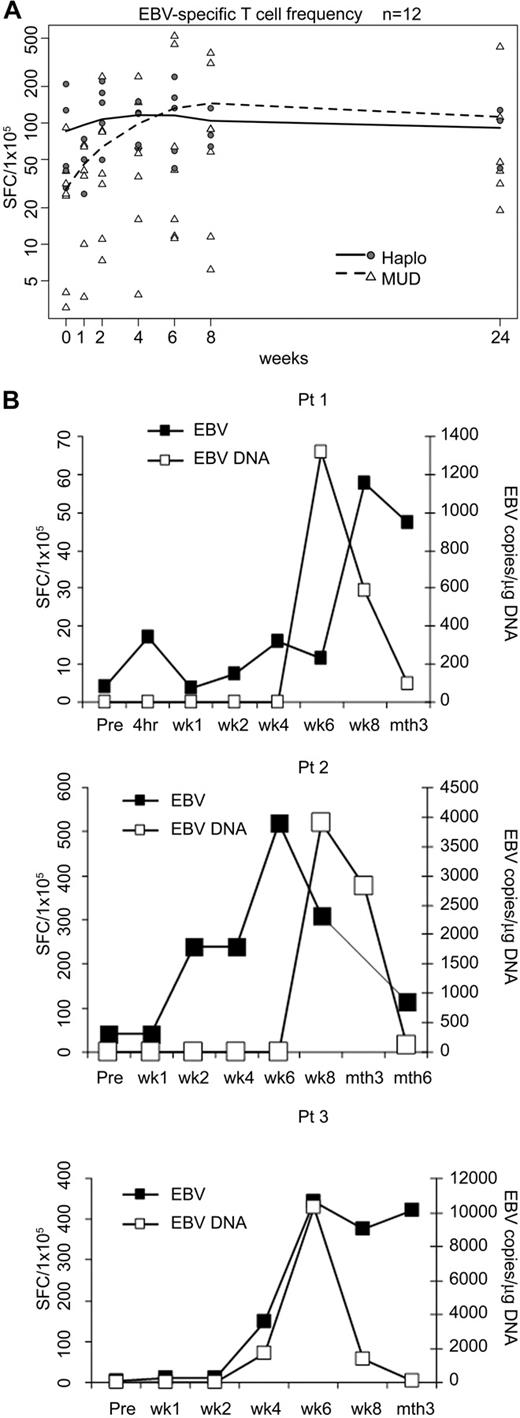
Measurement of the EBV-specific T-cell precursor frequency in patients' PBMCs before CTL infusion showed a median of 35 SFC/105 cells (range, 3-209 SFC/105cells) by ELIspot assay. Ten of the 12 evaluable patients studied (4 of 5 haploidentical and 6 of 7 matched unrelated recipients) had a marked rise in EBV-specific T cells within 2 weeks of CTL infusion (median, 85.3 SFC/105 cells; range, 7.3-240.7 SFC/105 cells; P = .02, exact one-sided matched-pair sign test). When we compared the haploidentical and unrelated transplantation recipients, there was no significant difference between the 2 groups (P = .76, Wilcoxon rank-sum test for the changes from week 0 to week 2; Figure 4A). We also obtained evidence for anti-EBV reactivity in vivo. Three MUD recipients (patients 1-3), all of whom received a dose of 5 × 106 CTL/m2, showed initially increased levels of EBV DNA. However, without additional CTL infusions or other treatment, virus load subsequently decreased to baseline in all 3 patients, coinciding with a marked expansion of EBV-reactive CTL precursors in vivo (Figure 4B). None of the haploidentical recipients reactivated EBV.
In vivo expansion and clinical benefits of EBV-specific T cells. (A) Frequencies of peripheral blood T cells responding to EBV-LCL by IFN-γ secretion before and after CTL infusion in 12 patients. Individual haploidentical recipients (n = 5) are represented by circles, and MUD recipients are represented by triangles (n = 7). Mean values are represented by lines: solid for haploidentical transplant recipients and dashed for MUDs. (B) Increase in the mean frequency of EBV-LCL-responsive peripheral blood T cells in patients 1, 2, and 3 is related to a decrease in EBV-DNA levels.
In vivo expansion and clinical benefits of EBV-specific T cells. (A) Frequencies of peripheral blood T cells responding to EBV-LCL by IFN-γ secretion before and after CTL infusion in 12 patients. Individual haploidentical recipients (n = 5) are represented by circles, and MUD recipients are represented by triangles (n = 7). Mean values are represented by lines: solid for haploidentical transplant recipients and dashed for MUDs. (B) Increase in the mean frequency of EBV-LCL-responsive peripheral blood T cells in patients 1, 2, and 3 is related to a decrease in EBV-DNA levels.
Adenoviral specificity
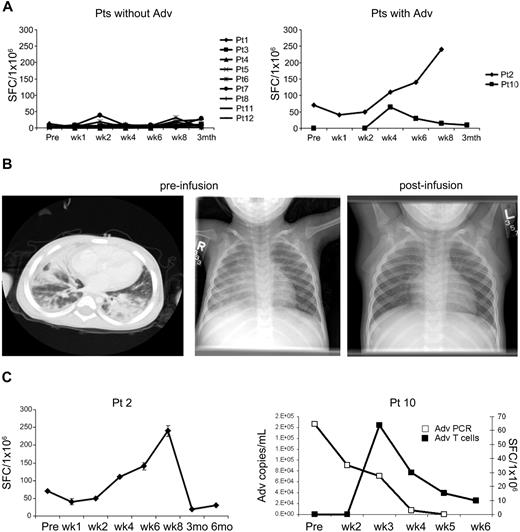
Twelve evaluable patients had a median of 5 SFC/105 cells (range, 0-70 adenovirus-specific SFC/105 cells) before the CTL infusion. In contrast to the EBV-specific T cells, there was no significant increase in the precursor frequency of adenovirus-specific cells, except in the 2 patients (patient 2, MUD recipient; and patient 10, haploidentical recipient) who had evidence of adenovirus infection/disease (Figure 5A).
Effects of adenovirus-specific CTL in vivo. (A) ELIspot analysis of the frequency of peripheral blood T cells responding to irradiated Ad5f35-infected donor PBMCs by IFN-γ secretion before and after CTL infusion in patients without (n = 9; left panel) and with (n = 2; right panel) adenovirus infection at the time of receiving CTL. (B) Imaging studies are shown before and after CTL in a patient (patient 2) with adenovirus pneumonia who had a complete clinical response after T-cell infusion. A chest CT scan and chest radiograph demonstrate bilateral interstitial infiltrates. The follow-up chest radiograph 2 weeks after CTL infusion is reported as normal (C). Marked increase in adenovirus-specific T-cell precursor frequency in patient 2, and marked decrease in adenoviral load in stool from patient 10 after CTL infusion and clearance of virus coincident with a transient increase in adenovirus-specific T cells.
Effects of adenovirus-specific CTL in vivo. (A) ELIspot analysis of the frequency of peripheral blood T cells responding to irradiated Ad5f35-infected donor PBMCs by IFN-γ secretion before and after CTL infusion in patients without (n = 9; left panel) and with (n = 2; right panel) adenovirus infection at the time of receiving CTL. (B) Imaging studies are shown before and after CTL in a patient (patient 2) with adenovirus pneumonia who had a complete clinical response after T-cell infusion. A chest CT scan and chest radiograph demonstrate bilateral interstitial infiltrates. The follow-up chest radiograph 2 weeks after CTL infusion is reported as normal (C). Marked increase in adenovirus-specific T-cell precursor frequency in patient 2, and marked decrease in adenoviral load in stool from patient 10 after CTL infusion and clearance of virus coincident with a transient increase in adenovirus-specific T cells.
Patient 2 received a MUD transplant and developed progressive adenoviral pneumonia approximately 130 days later. The patient received CTL at a dose of 5 × 106 CTL/m2 at day 150 after HSCT. At the time of CTL administration, the patient had extensive bilateral interstitial infiltrates on chest CT and x-ray (Figure 5B) and required mechanical ventilation, having failed to clear his infection with Cidofovir treatment. However, within 2 weeks of receiving CTL, the viral load, which was more than 1000 adenovirus copies/mL in his bronchial lavage before CTL infusion, had cleared, with a corresponding increase in the frequency of adenovirus-specific precursors detectable in the postinfusion blood samples (Figure 5C). Subsequently, the patient was extubated with normalization of his chest radiograph and remains well 3 years later (Figure 5B). Patient 10 demonstrated antiviral activity from infused CTL after haploidentical transplantation. This patient had developed persistent, unresponsive adenovirus infection in his gastrointestinal tract for 5 weeks before CTL. There was a rise in adenovirus-specific T-cell precursor frequency in the peripheral blood (Figure 5C), followed by clearance of virus from stool and resolution of symptoms.
High-risk recipients of T cell–depleted alternative donor transplants did not develop adenovirus infection after CTL infusion
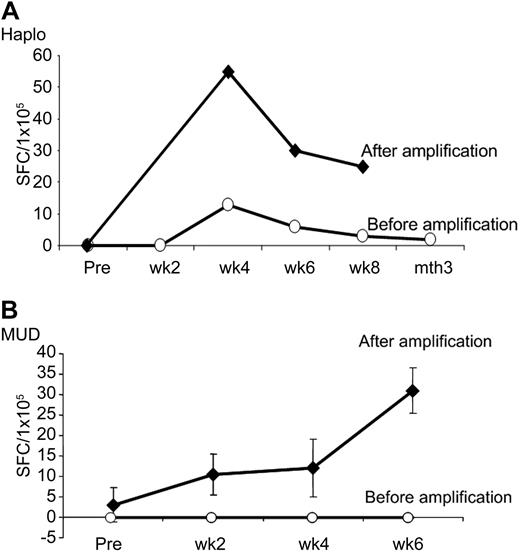
Although we only detected increases in adenovirus-specific T cells in the peripheral blood of our haploidentical and unrelated donor recipients with active adenovirus infections, none of the other 11 patients developed adenoviral infection or disease. This is compared with an expected incidence of 68% in pediatric subjects receiving similar transplants in the absence of CTL administration therapy.22 Because this unexpectedly low incidence of adenovirus infection was consistent with a protective effect from the infused cells, albeit at levels that were below the limit of detection of the ELIspot assay, we further analyzed PBMCs before and after infusion. We stimulated these PBMC samples in vitro with adenovirus antigens to amplify the frequency of adenovirus-reactive T cells and 9 days later analyzed the adenovirus-specific response using IFNγ ELIspot. As shown in Figure 6, we successfully amplified adenovirus-specific T cells exclusively from the postinfusion time points. Adenovirus-specific T cells were detectable for all available time points: at least 8 weeks in the haploidentical recipient and for 6 weeks in the matched unrelated recipient. Taken together, the data in Figures 5A and 6 suggest that, although infused CTLs require the presence of antigen in vivo to expand to levels that are detectable using conventional screening assays, the cells nonetheless persist for at least 8 weeks in all recipients, irrespective of whether the recipients are HLA-matched or mismatched, can be amplified by exposure to adenovirus, and may serve a protective function against adenoviral disease.
Amplification of adenovirus-specific T cells in vitro. (A) PBMCs from a haploidentical transplant recipient without adenovirus infection were stimulated in vitro with adenovirus antigens to amplify the frequency of adenovirus-reactive T cells and reanalyzed 9 days later by IFN-γ ELIspot. (B) Amplification of adenovirus-specific T cells from a MUD recipient without infection showing an increase in the adenovirus-specific T-cell precursor frequency using IFN-γ ELIspot.
Amplification of adenovirus-specific T cells in vitro. (A) PBMCs from a haploidentical transplant recipient without adenovirus infection were stimulated in vitro with adenovirus antigens to amplify the frequency of adenovirus-reactive T cells and reanalyzed 9 days later by IFN-γ ELIspot. (B) Amplification of adenovirus-specific T cells from a MUD recipient without infection showing an increase in the adenovirus-specific T-cell precursor frequency using IFN-γ ELIspot.
Discussion
We have produced monocultures of CTLs with specificity for both EBV and adenovirus using monocytes and EBV-LCLs modified with an adenovirus vector as APCs, and infused them into recipients of haploidentical or unrelated HSCT. Although the EBV-specific T-cell component routinely expanded after infusion, evidence for expansion of the adenovirus-specific component was observed only in patients with active infection, despite the high frequency of adenoreactive T cells in the infused CTL lines. However, adenovirus-specific T cells could be detected after a single ex vivo activation and expansion step, and no patient who received CTLs developed an adenovirus infection. These 2 facts suggested that the adenovirus-specific component did persist, perhaps with a different circulation pattern from that of EBV-specific T cells or simply with a lower frequency.26,27 The CTLs proved safe and effective because none of the patients developed GVHD, even when the donor was haploidentical to the recipient, and were functional in EBV and adenovirus infected persons, reducing viral load in association with resolution of virus-associated symptoms.
One of the objectives of the current study was to discover whether CTL therapy targeting EBV and adenovirus could be safe in the pediatric haploidentical donor transplantation setting. Haploidentical transplants, frequently from parental donors to a child recipient, are often mismatched at as many as 5 loci. Although this option means that nearly all pediatric patients have an immediately available donor, the immunologic consequences of crossing the major histocompatibility barrier, namely, GVHD, graft rejection, and delayed or incomplete immune reconstitution, are significant risk factors. Although T-cell depletion and reduced-intensity conditioning have significantly decreased GVHD and transplantation-related mortality, posttransplantation infectious complications, because of significantly delayed recovery of antigen-specific T cells,13 remain a critical barrier to long-term treatment success. Adenovirus infections are particularly problematic in the pediatric haploidentical transplantation setting and are associated with significant morbidity and mortality.1,9-11,28-32 However, because adenovirus disease largely affects nonhematopoietic cells of recipient origin,33,34 it was unclear whether there would be sufficient antiviral reactivity through the shared HLA alleles within the donor-derived CTL lines to produce in vivo efficacy. Six patients received bivirus-specific CTL from haploidentical donors at cell doses ranging from 1.5 × 107 T cells/m2 (equivalent to 5 × 105 cells/kg, 1 patient) to 1.35 × 108 T cells/m2 (equivalent to 4.5 × 106 cells/kg, 5 patients). We saw no evidence of dose-limiting toxicity associated with the infusion of these donor-derived T cells. Importantly, there was no development of de novo GVHD in the patients after CTL infusion, demonstrating the safety of these in vitro expanded bivirus-specific T cells. In contrast, several studies have reported that approximately 40% of patients receiving a dose of 105 unmanipulated T cells/kg develop grade 2 or higher GVHD after infusion,35,36 and a recent study from our group reported GVHD in 2 of 16 haploidentical transplantation recipients who received allodepleted T cells at doses of 104 to 105 cells/kg.37 Thus, ex vivo expanded bivirus-specific T cells appear to be a safer alternative to either donor lymphocyte infusion or allodepleted T cells because higher doses, which are exquisitely virus-specific and are not cross-reactive with recipient alloantigens,38 can be safely infused and do not induce toxicity, even in the partially HLA-matched setting.
We were concerned that our original trivirus-specific CTL products,7 which contained only a minor component of adenovirus-reactive T cells, would not confer effective immunity to pediatric recipients of unrelated or haploidentical grafts, who were at a particular high risk of adenovirus infections.1,8-11,28,29,39,40 We hypothesized that the low frequency of adenovirus-specific T cells within trivirus-specific CTLs was the result of a combination of factors, in particular to the low frequency of circulating adenovirus-specific T cells relative to EBV- and CMV-specific T cells in healthy donors,41-43 and to competition from the strong immunodominant CMV and EBV antigens for presentation by HLA molecules in the antigen-presenting cells.44,45 Indeed, removal of the CMV component resulted in an increase in both the EBV- and adenovirus-specific T-cell component within our CTL lines, resulting in roughly equivalent numbers of virus reactive cells (P = .87), and a significantly higher frequency of adenovirus-specific T cells than that contained within the trivirus CTL lines infused in our previous study (P = .006).7
We next addressed whether infusion of lines containing increased frequencies of adenovirus-reactive T cells would translate to their consequential detection in unmanipulated postinfusion blood samples. However, we saw no evidence of adenovirus-reactive cells after infusion except in patients with adenovirus infection or disease at the time of CTL therapy, and this held true for both haploidentical and unrelated transplant recipients. We cannot exclude that this lack of detection in peripheral blood may reflect the altered migration pattern of T cells specific for nonlymphoid tissues.26,27,46 Nevertheless, the ability to detect a significant expansion of adenovirus-specific T cells in the periphery after adoptive T-cell transfer clearly requires the presence of antigen in vivo.47
None of the treated patients developed a de novo adenovirus infection after infusion compared with an infection rate of 68% in pediatric patients who did not receive CTL therapy.13 These results suggest that the ability to expand adenovirus-specific T cells to levels measurable in peripheral blood is dependent more on the presence of antigen rather than on the dose infused. However, even low doses (5 × 106-1.35 × 108/m2) of polyclonal CTL, derived from peripheral blood T cells with both effector and central memory characteristics, are able to survive and persist after infusion and mediate antiviral protection in the lymphopenic host.48 In support of this supposition, we were successfully able to demonstrate persistence of adenovirus-specific T cells at all postinfusion time points tested (at least 8 weeks after adoptive transfer) by performing in vitro amplification experiments using postinfusion blood samples from both haploidentical and unrelated recipients.
Extensive epitope analysis of the immune response directed against hexon demonstrated that the infused lines recognized multiple CD4+ and CD8+ T-cell epitopes.12 This breadth of reactivity should ensure that virus-specific T-cell activity will be mediated through both shared and nonshared alleles; and indeed, the infused CTL showed evidence of in vivo efficacy in both haploidentical and unrelated transplant recipients. Notably, all persons with evidence of active EBV (3 MUD recipients) or adenoviral infection (one haploidentical and one MUD recipient) had a relatively rapid reduction in viral titer and resolution of disease symptoms (within 2-6 weeks), which coincided with the expansion of virus-specific CTLs. In contrast to the lack of adenovirus and EBV infections, 8 of the 11 patients who received bivirus-specific CTL as prophylaxis developed multiple other viral infections, which persisted for up to 16 weeks (Table 3). These prolonged viral infections and reactivations confirm that virus-specific T-cell immunity was generally impaired in these patients and suggest that the administration of bivirus-specific CTL had specifically improved immunity to EBV and adenovirus only.
Outcomes in patients with viral infections
| UPN . | Donor . | Day of CTL . | Active viral infections . | Treatment . | Time to viral clearance after treatment (or diagnosis), wk . |
|---|---|---|---|---|---|
| 1216 | MUD | 104 | EBV BK virus | CTL None | 4-616 (after diagnosis) |
| 1217 | MUD | 150 | EBV Adv | CTL CTL | 6 2 |
| 1218 | MUD | 134 | EBV BK virus | CTL None | 2 16 (after diagnosis) |
| 1244 | HAPLO | 77 | CMV VZV | Ganciclovir Acyclovir | 3 6 |
| 1252 | MUD | 53 | HSV Parainfluenza | Acyclovir None | 16 8 (after diagnosis) |
| 1265 | MUD | 80 | BK virus | None | Unknown; positive > 4 (after diagnosis) |
| 1287 | MUD | 40 | BK virus | None | Unknown; positive > 4 (after diagnosis) |
| 1352 | HAPLO | 95 | Adv | CTL | 4 |
| 1358 | HAPLO | 86 | CMV | Foscarnet | 12 |
| 1377 | HAPLO | 77 | CMV Parainfluenza | Foscarnet None | 8 3 (after diagnosis) |
| UPN . | Donor . | Day of CTL . | Active viral infections . | Treatment . | Time to viral clearance after treatment (or diagnosis), wk . |
|---|---|---|---|---|---|
| 1216 | MUD | 104 | EBV BK virus | CTL None | 4-616 (after diagnosis) |
| 1217 | MUD | 150 | EBV Adv | CTL CTL | 6 2 |
| 1218 | MUD | 134 | EBV BK virus | CTL None | 2 16 (after diagnosis) |
| 1244 | HAPLO | 77 | CMV VZV | Ganciclovir Acyclovir | 3 6 |
| 1252 | MUD | 53 | HSV Parainfluenza | Acyclovir None | 16 8 (after diagnosis) |
| 1265 | MUD | 80 | BK virus | None | Unknown; positive > 4 (after diagnosis) |
| 1287 | MUD | 40 | BK virus | None | Unknown; positive > 4 (after diagnosis) |
| 1352 | HAPLO | 95 | Adv | CTL | 4 |
| 1358 | HAPLO | 86 | CMV | Foscarnet | 12 |
| 1377 | HAPLO | 77 | CMV Parainfluenza | Foscarnet None | 8 3 (after diagnosis) |
UPN indicates unique patient number; CTL, cytotoxic T lymphocyte; MUD, matched unrelated donor; EBV, Epstein-Barr virus; Adv, adenovirus; CMV, cytomegalovirus; VZV, varicella-zoster virus; HSV, herpes simplex virus; and HAPLO, haploidentical donor.
In conclusion, we routinely produced CTL with simultaneous specificity for adenovirus and EBV from donors of haploidentical and unrelated transplants. We demonstrated that these CTLs can be safely infused to MUD as well as high-risk haploidentical HSCT recipients, in whom HLA matching varied from 5 to 8 of the 10 loci, without inducing GVHD. Irrespective of the degree of mismatch, the infused cells mediated immediate therapeutic effector function and provided prolonged protection when used as prophylaxis. Thus, CTL directed against both lytic and latent clinically relevant viruses can mediate effective and protective in vivo effects in pediatric allogeneic HSCT recipients irrespective of the graft source. Our findings advocate for increasing the spectrum of viruses, which can be targeted using this type of therapeutic approach.49,50 Furthermore, the availability of virus-specific T cells for adoptive transfer may significantly increase the safety of haploidentical transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yu-Feng Lin for research coordination and T. Lopez and staff in the Good Manufacturing Practice facilities for assisting in CTL preparation and quality assurance.
This work was supported by a Specialized Centers for Cell-based Therapy Grant, National Institutes of Health (NIH-NHLBI 1 U54 HL081007), the National Gene Vector Laboratories (NIH-NCRR U42 RR16578), the General Clinical Research Center at Baylor College of Medicine (RR00188), the Dan L. Duncan Cancer Center, a Doris Duke Distinguished Clinical Scientist award (H.E.H.), and an Amy Strelzer Manasevit Scholar Award (A.M.L).
National Institutes of Health
Authorship
Contribution: C.M.R., H.E.H., M.K.B., C.M.B., and A.M.L. developed and designed the study; C.M.B. and A.M.L. were the principal investigators on the clinical trial and developed the method for generating bivirus-specific CTLs; A.M.L. grew the CTL lines in the GMP facility; A.C., G.D.M., C.R.C., P.J.H., and A.M.L. performed the PCR and immune reconstitution studies; R.A.K., G.D.M., A.A.K.-N., C.M.B., and K.S.L. cared for the transplantation subjects enrolled in this study; H.L. provided statistical support; A.P.G. supervised CTL preparation and quality assurance; and C.M.R., H.E.H., M.K.B., C.M.B., and A.M.L. contributed to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ann M. Leen, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin St, MC 3-3320, Houston, TX 77030; e-mail: amleen@txccc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal