Abstract
BCL10, required for nuclear factor κB (NF-κB) activation during antigen-driven lymphocyte responses, is aberrantly expressed in mucosa-associated lymphoid tissue-type marginal zone (MZ) lymphomas because of chromosomal translocations. Eμ-driven human BCL10 transgenic (Tg) mice, which we created and characterize here, had expanded populations of MZ B cells and reduced follicular and B1a cells. Splenic B cells from Tg mice exhibited constitutive activation of both canonical and noncanonical NF-κB signaling pathways is associated with increased expression of NF-κB target genes. These genes included Tnfsf13b, which encodes the B-cell activating factor (BAFF). In addition, levels of BAFF were significantly increased in sera from Tg mice. MZ B cells of Tg mice exhibited reduced turnover in vivo and enhanced survival in vitro, indicative of lymphoaccumulation rather than lymphoproliferation as the cause of MZ expansion. In vivo antibody responses to both T-independent, and especially T-dependent, antigens were significantly reduced in Tg mice. Mortality was accelerated in Tg animals, and some mice older than 8 months had histologic and molecular findings indicative of clonal splenic MZ lymphoma. These results suggest that, in addition to constitutive activation of BCL10 in MZ B cells, other genetic factors or environmental influences are required for short latency oncogenic transformation.
Introduction
The BCL10 gene was cloned from the t(1;14)(p22;q32) chromosomal translocation breakpoint associated with B-cell lymphomas of mucosa-associated lymphoid tissue (MALT lymphomas) that results in overexpression of normal BCL10 protein in B cells.1 Studies of BCL10-deficient mice (Bcl10−/−) identified essential roles for BCL10 in antigen (Ag) receptor-mediated nuclear factor κB (NF-κB) signal transduction in B and T cells.2,3 Signals initiated by B-cell receptor (BCR) ligation activate the lipid raft-associated protein, CARD11 (CARMA1). CARD11 recruits BCL10 to the rafts, which then binds MALT1 to form the “CARMA1-BCL10-MALT1 signalosome.”4 This complex activates IKBKG (IKKγ/NEMO), promoting activation and nuclear transport of NF-κB p50 and p65. BCL10 also plays important roles in B-cell development. The phenotypes of mice with null alleles of Bcl10,2,3 Carma1,5 Malt1,6,7 Nfκb1 (p50), and Rel (c-Rel) indicate critical roles for these proteins in generation of marginal zone (MZ), B1a and, to a lesser extent, follicular (FO) B2 cells.8,9
Aside from translocations affecting BCL10, approximately 40% to 50% of MALT lymphomas bear 1 of 2 translocations involving the MALT1 gene: t(11;18)(q21;q21), which generates a chimeric API2-MALT1 fusion protein, and t(14;18)(q32;q21), which results in overexpression of normal MALT1 protein.10,11 Aside from activating NF-κB signaling,12,13 the contributions of deregulated API2-MALT1 or MALT1 to lymphomagenesis is unknown, although changes in the cellular distributions of MALT1 and BCL10 may be important. Indeed, aberrant nuclear localization of BCL10 occurs in MALT lymphomas expressing the API2-MALT1 fusion protein or overexpressing BCL10 but not in cases overexpressing MALT1.1,14
Here, we engineered expression of a human BCL10 transgenic (Tg) in B cells to recapitulate the consequences of aberrant BCL10 expression associated with the t(1;14)(p22;q32) translocation. These mice exhibited constitutive activation of both the canonical and noncanonical NF-κB pathways together with a substantial increase in MZ B cells. Consistent with constitutive NF-κB signaling, Tg B cells aberrantly expressed a number of NF-κB targets, most notably Tnfsf13b, which encodes B-cell activating factor (BAFF).15 Furthermore, some older mice developed clonal splenic MZ lymphomas.
Methods
Generation of BCL10 Tg mice
Full-length human BCL10 cDNA flanked by an EμSRα enhancer/promoter and a polyadenylation signal element (Figure 1A) was used to generate Tg mice in strain FVB.16 Tg expression was verified by Western blotting using a mouse monoclonal Ab (mAb) specific for human BCL10 (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). BCL10 Tg+ mice were bred to generate Tg/+, Tg/Tg, and wild-type (WT) animals. Animal experiments were approved by the Animal Care and Use Committees of St Jude Children's Research Hospital and National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Histologic and molecular studies
Approximately 20 each of WT, Tg/+, and Tg/Tg mice were observed long term and were examined by necropsy when moribund. At necropsy, tissues were fixed in formalin for later preparation of slides stained with H&E and for immunohistochemistry (IHC).17 Histologic diagnoses followed the nomenclature of the Bethesda Proposals.18 Portions of spleens were frozen for preparation of DNA. Southern blots of DNAs digested with EcoRI and hybridized with a JH probe were performed as described.19
Flow cytometry
Single-cell suspensions were stained with mAb conjugated to FITC, PE, PerCP, APC, or biotin (BD Biosciences; supplemental Table 1), assayed on a FACSCalibur (Becton Dickinson). The data were analyzed with FlowJo (TreeStar Inc) or WinMDI (The Scripps Institute) software.
Spleen B cells were negatively enriched using magnetic-activated Dynal beads (Invitrogen). To purify FO and MZ B cells, pre-enriched B cells were stained with mAb to CD21, CD23, and B220 and sorted with a FACSAria-Green (BD Biosciences) or a MoFlo cell sorter (Dako North America Inc). Purity of the sorted populations was greater than 95%.
Survival and proliferation assays
Purified B cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured in RPMI 1640 medium with 10% FBS at 1 to 2 × 106/mL in 24-well plates. Cells were incubated with 10 μg/mL of goat F(ab′)2 anti–mouse IgM Ab (Southern Biotechnology Associates) or 1 μg/mL of LPS (Sigma-Aldrich) for 72 hours, stained with APC-conjugated annexin V and 7-AAD, and analyzed by flow cytometry.
Immunizations and ELISA
Mice injected intraperitoneally with 50 μg of TNP-LPS or 25 μg of TNP-Ficoll (Biosearch Technologies) were bled at 7 days. Mice immunized intraperitoneally with 100 μg of TNP-KLH (Biosearch Technologies) in complete Freund adjuvant were bled and boosted intraperitoneally at day 21 with 100 μg of Ag in incomplete Freund adjuvant with serum collected 14 days later. Sera were tested for TNP-specific Ab by enzyme-linked immunosorbent assay (ELISA).3 Other mice were immunized with 100 μg of TNP-KLH in alum. Serum BAFF levels were determined using a mouse BAFF/BLyS/TNFSF13B immunoassay kit (R&D Systems).
Western blot analysis
Total cell lysates were resolved by SDS–polyacrylamide gel electrophoresis and blotted with a primary Ab, followed by a HRP-labeled secondary Ab (R&D Systems). Cytoplasmic and nuclear fractions were prepared using a NE-PER nuclear and cytoplasmic extraction kit (Pierce Chemical). Protein bands were visualized using a Super Signal West pico detection kit (Pierce Chemical). Detection of human BCL10 protein was performed by immunoblotting with a rabbit mAb to human BCL10 that does not recognize mouse BCL10 (supplemental Table 1).
Quantitative real-time RT-PCR
Quantitative real-time reverse transcription polymerase chain reaction (qPCR) was performed using 384-well plates containing predetermined primer pairs representing genes known to be cancer-related and/or important for lymphoid cell development and function (Bar Harbor BioTechnology) with the gene list described previously.20 PCR amplification was performed using regular SYBR-Green reagents (Applied Biosystems) and analyzed by a global pattern recognition algorithm as recently modified (http://array.lonza.com/gpr/).21
Results
Generation of BCL10 Tg mice
To better understand the contributions of BCL10 to normal B-cell biology and pathology, we generated Tg mice (Figure 1A) harboring a full-length, normal human BCL10 cDNA directed by EμSRα regulatory sequences that drive expression in B and T cells and are active in the earliest committed B-cell progenitors.16 Human and mouse BCL10 proteins are 91% identical and thus probably share functional attributes. Two founder lines had no gross anatomic abnormalities, had normal life spans and fertility, and were similar phenotypically. These studies used mice from the line with higher Tg copy number and level of BCL10 expression.
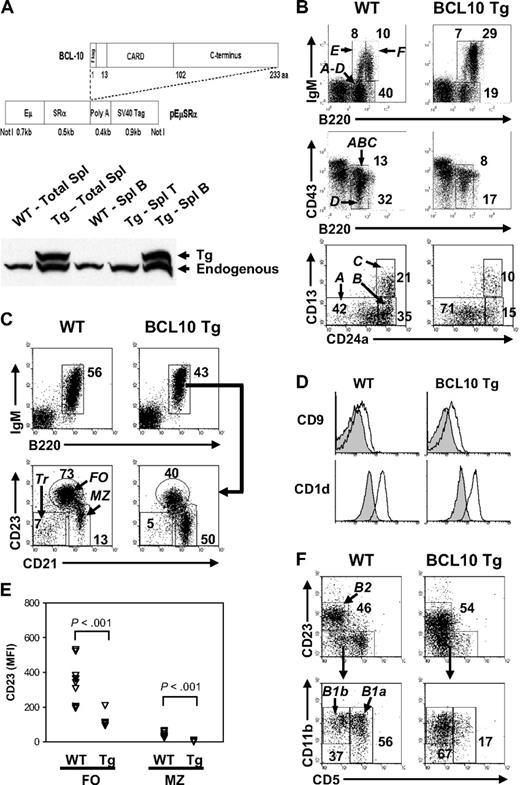
Generation of BCL10 Tg mice and analysis of B-cell subsets. (A) BCL10 transgenic (Tg) construct and expression in spleen by Western blotting. The full-length, wild-type (WT) human BCL10 cDNA was placed under control of the EμSRα enhancer/promoter upstream of a polyA signal element16 with an N-terminal FLAG tag fused to the BCL10 protein. Expression of Tg protein in spleen was examined using cell lysates from total splenocytes or purified splenic T and B cells from WT and BCL10 Tg mice analyzed by immunoblotting with an anti-BCL10 Ab that recognizes both human and mouse proteins (a rabbit polyclonal generated by the Morris Laboratory). The human Tg BCL10 protein migrates slightly slower than mouse BCL10 because of the presence of the N-terminal FLAG tag. (B) Distribution of Hardy fraction (Fr) A to F B cells in BM of BCL10 Tg and WT littermates. Fluorescence-activated cell sorting (FACS) analyses of BM B-cell subsets. Cells were gated on lymphocytes (top), IgM− lymphocytes (middle), or B220+CD43+ lymphocytes (bottom). The numbers are percentages of cells falling within each gate. (C) Distribution of transitional (Tr), follicular (FO), and marginal zone (MZ) B cells in the spleen. The cells were gated on lymphocytes. The numbers are percentages of cells falling within each gate and are representative of analyses of 6 Tg and WT mice. (D) Expression of CD9 and CD1d on FO (filled line) and MZ (open line) B cells. The gates used for defining FO and MZ B cells are shown in panel C. All histograms are gated on CD19+ cells. (E) FACS comparisons of mean fluorescence intensities (MFIs) of CD23 expression on FO and MZ B cells from Tg and WT mice. Each triangle represents data from analysis of cells from an individual mouse of the indicated genotype. Note the marked decrease in CD23 expression on the Tg FO B cells (P < .001). (F) Analysis of B2, B1a, and B1B cells in the peritoneal cavity. The numbers are percentages of cells falling within each gate. All cells are gated on the CD19+ population.
Generation of BCL10 Tg mice and analysis of B-cell subsets. (A) BCL10 transgenic (Tg) construct and expression in spleen by Western blotting. The full-length, wild-type (WT) human BCL10 cDNA was placed under control of the EμSRα enhancer/promoter upstream of a polyA signal element16 with an N-terminal FLAG tag fused to the BCL10 protein. Expression of Tg protein in spleen was examined using cell lysates from total splenocytes or purified splenic T and B cells from WT and BCL10 Tg mice analyzed by immunoblotting with an anti-BCL10 Ab that recognizes both human and mouse proteins (a rabbit polyclonal generated by the Morris Laboratory). The human Tg BCL10 protein migrates slightly slower than mouse BCL10 because of the presence of the N-terminal FLAG tag. (B) Distribution of Hardy fraction (Fr) A to F B cells in BM of BCL10 Tg and WT littermates. Fluorescence-activated cell sorting (FACS) analyses of BM B-cell subsets. Cells were gated on lymphocytes (top), IgM− lymphocytes (middle), or B220+CD43+ lymphocytes (bottom). The numbers are percentages of cells falling within each gate. (C) Distribution of transitional (Tr), follicular (FO), and marginal zone (MZ) B cells in the spleen. The cells were gated on lymphocytes. The numbers are percentages of cells falling within each gate and are representative of analyses of 6 Tg and WT mice. (D) Expression of CD9 and CD1d on FO (filled line) and MZ (open line) B cells. The gates used for defining FO and MZ B cells are shown in panel C. All histograms are gated on CD19+ cells. (E) FACS comparisons of mean fluorescence intensities (MFIs) of CD23 expression on FO and MZ B cells from Tg and WT mice. Each triangle represents data from analysis of cells from an individual mouse of the indicated genotype. Note the marked decrease in CD23 expression on the Tg FO B cells (P < .001). (F) Analysis of B2, B1a, and B1B cells in the peritoneal cavity. The numbers are percentages of cells falling within each gate. All cells are gated on the CD19+ population.
Western blot analyses showed that the transgene was expressed in splenic B cells but not T cells (Figure 1A). The transgene was also expressed in the thymus (not shown), but there were no substantive alterations in the proportions of intrathymic or peripheral T-cell subsets (supplemental Figure 1A).
Splenomegaly and MZ expansion in Tg mice
At necropsy, nonlymphoid tissues of Tg/+ mice were grossly normal. Thymi and nodes were normal in size and histologically unremarkable, and bone marrow (BM) was of normal cellularity. Young (6-8 weeks old) Tg/+ mice exhibited splenomegaly with spleen weights approximately 40% greater than for WT mice (P < .001) and had a significant increase in spleen-to-body weight ratios (P < .05; Table 1). Histologically, spleens of young mice had significant enlargement of the MZ that, with age, caused a progressive compression of both the white and red pulp. IHC studies confirmed expansion of the B220+ B-cell population and compaction of the CD3+ T-cell population around central arterioles (supplemental Figure 1B). Thus, constitutive overexpression of BCL10 in B cells resulted in splenomegaly with a substantial expansion of MZ B cells.
B-cell subpopulations in WT and BCL10 Tg mice
| . | WT . | BCL10 Tg . |
|---|---|---|
| Bone marrow (× 105/femur)* | ||
| B cells (B220+) | 27.7 ± 2.7 | 24.1 ± 1.8 |
| Fr A B cells (B220+CD43+CD24a−CD13−) | 2.1 ± 0.1 | 2.7 ± 0.3† |
| Fr B/C B cells (B220+CD43+CD24a+CD13+/−) | 1.9 ± 0.3 | 1.1 ± 0.2 |
| Fr D pre-B cells (B220+IgM−CD43−/lo) | 12.4 ± 1.0 | 7.0 ± 1.1‡ |
| Fr E immature B cells (B220loIgM+) | 4.4 ± 0.4 | 3.4 ± 0.4 |
| Fr F recirculating B cells (B220hiIgM+) | 6.7 ± 1.0 | 9.8 ± 1.0† |
| Spleen* | ||
| Weight (mg) | 100 ± 10.0 | 140 ± 10.0‡ |
| Spleen/body weight ratio | 0.36 ± 0.03 | 0.48 ± 0.04† |
| Total B cells (% of total) | 54.5 ± 1.9 | 47.7 ± 4.0 |
| Transitional (B220+IgM+CD23−CD21−) | 2.9 ± 0.2 | 1.7 ± 0.2‡ |
| Follicular (B220+IgM+CD23+CD21−/lo) | 29.6 ± 1. | 19.1 ± 1.9‡ |
| MZ (B220+IgM+CD23−CD21+) | 9.4 ± 0.6 | 14.7 ± 1.6‡ |
| Peritoneum (× 105)§ | ||
| B cells (CD19+) | 4.5 ± 1.2 | 9.8 ± 1.8† |
| B2 (CD19+CD23+CD5−) | 2.9 ± 0.1 | 5.3 ± 0.7† |
| B1a (CD19+CD23−CD5+) | 1.1 ± 0.2 | 0.2 ± 0.1‡ |
| B1b (CD19+CD23−CD5−CD11b+) | 1.2 ± 0.3 | 2.8 ± 0.9 |
| . | WT . | BCL10 Tg . |
|---|---|---|
| Bone marrow (× 105/femur)* | ||
| B cells (B220+) | 27.7 ± 2.7 | 24.1 ± 1.8 |
| Fr A B cells (B220+CD43+CD24a−CD13−) | 2.1 ± 0.1 | 2.7 ± 0.3† |
| Fr B/C B cells (B220+CD43+CD24a+CD13+/−) | 1.9 ± 0.3 | 1.1 ± 0.2 |
| Fr D pre-B cells (B220+IgM−CD43−/lo) | 12.4 ± 1.0 | 7.0 ± 1.1‡ |
| Fr E immature B cells (B220loIgM+) | 4.4 ± 0.4 | 3.4 ± 0.4 |
| Fr F recirculating B cells (B220hiIgM+) | 6.7 ± 1.0 | 9.8 ± 1.0† |
| Spleen* | ||
| Weight (mg) | 100 ± 10.0 | 140 ± 10.0‡ |
| Spleen/body weight ratio | 0.36 ± 0.03 | 0.48 ± 0.04† |
| Total B cells (% of total) | 54.5 ± 1.9 | 47.7 ± 4.0 |
| Transitional (B220+IgM+CD23−CD21−) | 2.9 ± 0.2 | 1.7 ± 0.2‡ |
| Follicular (B220+IgM+CD23+CD21−/lo) | 29.6 ± 1. | 19.1 ± 1.9‡ |
| MZ (B220+IgM+CD23−CD21+) | 9.4 ± 0.6 | 14.7 ± 1.6‡ |
| Peritoneum (× 105)§ | ||
| B cells (CD19+) | 4.5 ± 1.2 | 9.8 ± 1.8† |
| B2 (CD19+CD23+CD5−) | 2.9 ± 0.1 | 5.3 ± 0.7† |
| B1a (CD19+CD23−CD5+) | 1.1 ± 0.2 | 0.2 ± 0.1‡ |
| B1b (CD19+CD23−CD5−CD11b+) | 1.2 ± 0.3 | 2.8 ± 0.9 |
The cell numbers (mean ± SE) were calculated from 6 to 9 mice based on cell count and flow cytometric analysis using gating schemes illustrated in Figure 1.
WT indicates wild-type; Tg, transgenic; Fr, fraction; and MZ, marginal zone.
Bone marrow (n = 9) and BCL10 Tg (n = 7).
P < .05 compared with WT controls.
P <.01 compared with WT controls.
Bone marrow (n = 7) and BCL10 Tg (n = 6).
B-cell development in Tg mice is altered at multiple stages
We used flow cytometry to characterize B-cell subsets in the BM and peripheral lymphoid compartments of Tg/+ and WT mice. Comparative analyses of cells from single Tg and WT mice are shown in Figure 1 with data from multiple mice summarized in Table 1. In the BM of Tg/+ mice, the frequency and absolute numbers of early B-cell progenitors in Hardy Fraction (Fr) A (pre-pro-B cells) were increased (Figure 1B; Table 1), whereas cells from later stages of differentiation, including Fr B (early pro-B), C (late pro-B), D (pre-B), and E (immature B) were decreased. Recirculating mature B cells (Fr F), however, were increased (Figure 1B; Table 1). The recirculating cells were phenotypically abnormal because they expressed significantly higher levels of surface IgM and lower levels of CD23 (supplemental Figure 1C).
The proportions of B cells in spleens of young WT and Tg/+ mice were similar. In Tg/+ spleens, the frequencies of transitional and FO B cells were reduced by 41% and 35%, respectively, whereas the proportions of MZ B cells were increased by as much as 4-fold (Figure 1C), with an average increase of 1.56-fold (Table 1). The CD21hiCD23lo/− MZ B cells from Tg/+ mice were normal for expression of the MZ B-cell markers, CD9 and CD1d (Figure 1D), indicating that they were truly MZ B cells. Similar to recirculating BM B cells, expression of CD23 on FO and MZ B cells of Tg/+ mice was significantly reduced (P < .001; Figure 1C,E). The total numbers of Tg/+ peritoneal B cells were increased approximately 2-fold because of increases in both the B2 and B1b populations with the numbers of CD5+ B1a cells significantly reduced (Figure 1F; Table 1). Thus, constitutive expression of BCL10 impaired development of early, FO, and B1a B cells but resulted in the expansion of the MZ B-cell subset.
Constitutive expression of BCL10 affects B-cell survival and proliferation
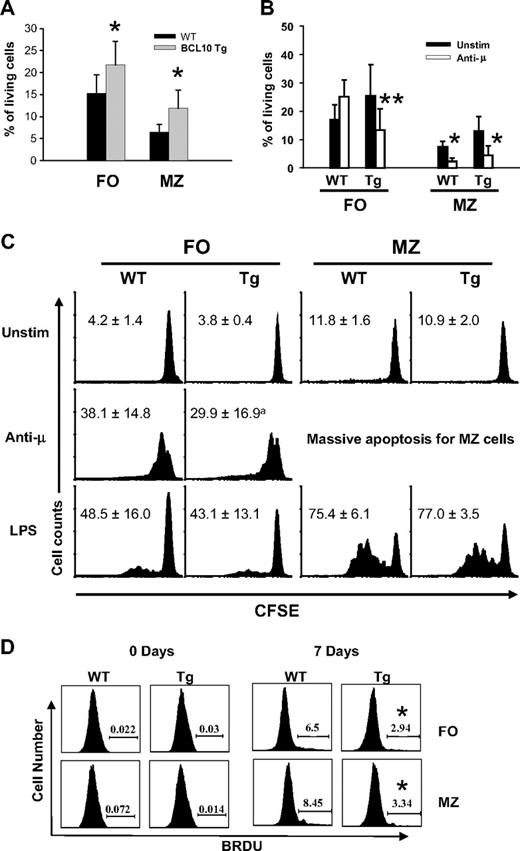
Analyses of sort-purified FO and MZ B cells cultured for 72 hours showed that survival of Tg/+ B cells of both types was significantly enhanced (Figure 2A). We also assessed cell survival after BCR ligation, which normally induces apoptosis of MZ B cells but proliferation of FO B cells.22 As expected, survival of MZ B cells from WT and Tg/+ mice was reduced after IgM crosslinking, but, unexpectedly, survival of Tg/+ FO B cells was also reduced. (Figure 2B). To further examine subset survival and proliferation, we studied cells labeled with CFSE after BCR ligation or stimulation with LPS. MZ cells from mice of both genotypes treated with anti-IgM underwent massive apoptosis (Figure 2C). Although FO B cells from WT mice proliferated in response to anti-IgM, many FO B cells from Tg/+ mice underwent apoptosis. Tg/+ cells that survived did proliferate, but to a somewhat lesser extent than those of WT mice, a difference that was statistically insignificant (P = .12; n = 3; Figure 2C). Notably, spontaneous proliferation of MZ B cells from mice of both genotypes was similar and higher than the low levels seen for FO cells.
Functional analysis of FO and MZ B cells of BCL10 Tg mice. (A) FACS-purified B cells were cultured for 72 hours, then stained for 7AAD and Annexin V detection, and viable cells were determined by FACS. The data (mean ± SEM) shown are from 4 independent experiments. *P < .05. (B) Apoptosis induced by BCR ligation. Purified B cells were stimulated with anti-IgM (10 μg/mL) for 72 hours, stained for 7AAD and annexin V detection, and analyzed by FACS. *P < .05, **P < .01. (C) Proliferation of FO and MZ B cells in response to stimulation with anti-IgM and LPS. Cells labeled with CFSE were cultured in the presence of F(ab)′2 anti-IgMμ (20 μg/mL) or LPS (1 mg/mL) for 72 hours. The cells were then stained to detect 7AAD and annexin V and analyzed by FACS, with the histograms gated on viable cells. The numbers are percentages of cells undergoing more than 2 divisions. Data (mean ± SE) are from 3 independent experiments; aP = .12. (D) B-cell proliferation in vivo. Mice were injected intraperitoneally with BrdU at 12-hour intervals for 7 days. Splenocytes were stained with Abs to B220, CD21, and CD23 to identify B-cell subsets, then fixed, permeabilized, and stained to assess incorporation of BrdU by FACS. Numbers (mean ± SD) indicate the percentages of BrdU+ cells from 5 to 6 mice in each group. *P < .05 compared with WT controls.
Functional analysis of FO and MZ B cells of BCL10 Tg mice. (A) FACS-purified B cells were cultured for 72 hours, then stained for 7AAD and Annexin V detection, and viable cells were determined by FACS. The data (mean ± SEM) shown are from 4 independent experiments. *P < .05. (B) Apoptosis induced by BCR ligation. Purified B cells were stimulated with anti-IgM (10 μg/mL) for 72 hours, stained for 7AAD and annexin V detection, and analyzed by FACS. *P < .05, **P < .01. (C) Proliferation of FO and MZ B cells in response to stimulation with anti-IgM and LPS. Cells labeled with CFSE were cultured in the presence of F(ab)′2 anti-IgMμ (20 μg/mL) or LPS (1 mg/mL) for 72 hours. The cells were then stained to detect 7AAD and annexin V and analyzed by FACS, with the histograms gated on viable cells. The numbers are percentages of cells undergoing more than 2 divisions. Data (mean ± SE) are from 3 independent experiments; aP = .12. (D) B-cell proliferation in vivo. Mice were injected intraperitoneally with BrdU at 12-hour intervals for 7 days. Splenocytes were stained with Abs to B220, CD21, and CD23 to identify B-cell subsets, then fixed, permeabilized, and stained to assess incorporation of BrdU by FACS. Numbers (mean ± SD) indicate the percentages of BrdU+ cells from 5 to 6 mice in each group. *P < .05 compared with WT controls.
Studies of Bcl10−/− mice showed that BCL10 is not required for LPS-induced proliferation of FO B cells but is required for the full activation of MZ B cells.3 In our studies, B cells from mice of both genotypes responded comparably to LPS (Figure 2C). Thus, although BCL10 deficiency impairs LPS-induced MZ B-cell proliferation, overexpression did not enhance this activity. In general, the responses to LPS or BCR crosslinking by MZ or FO B cells from WT and Tg/+ mice were remarkably similar, with MZ cells responding much more vigorously (Figure 2C). We conclude that in vitro resting FO and MZ B cells from Tg/+ mice have a survival advantage over cells from WT mice, but that FO B cells from Tg/+ mice are unusually sensitive to induction of apoptosis after BCR ligation.
To determine how these in vitro analyses relate to properties of B cells in vivo, we studied BrdU incorporation by B cells from mice injected with BrdU at 12-hour intervals for 1 week (Figure 2D). Interestingly, the frequencies of BrdU+ cells among FO and MZ B cells of Tg/+ mice were significantly lower than for cells from WT mice (P < .05). These results indicate that MZ B-cell expansion in Tg/+ mice is a cumulative process involving cells with reduced proliferative activity but enhanced survival potential. They also suggest that the reduced size of the Tg/+ FO B-cell population may be due to an unusual susceptibility to BCR-induced apoptosis.
BCL10 overexpression induces constitutive activation of NF-κB
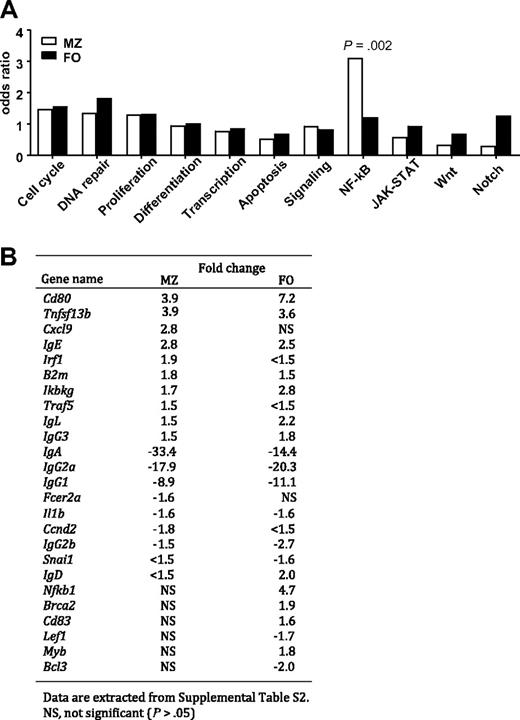
BCL10, interacting with CARMA1 and MALT1, activates IKK complexes, activating the canonical NF-κB pathway.23,24 To further understand the consequences of BCL10 overexpression, we used qPCR arrays to profile expression of 384 genes with known involvement in cancer, with importance for lymphocyte development and function, or with both in sort-purified MZ and FO B cells of Tg/+ and WT mice. One hundred eight genes (28.1%) with significantly altered expression (P < .05) were identified,20,21 functional categorized by gene ontology, and analyzed for enrichment within each category by Fisher exact test (Figure 3A). There was significant enrichment (P = .002) specifically for genes identified as direct transcriptional targets of NF-κB (listed at http://people.bu.edu/gilmore/nf-kb/index.html). Among 62 NF-κB targets on the array, 25 (40.3%) were significantly changed in expression in comparisons of the MZ or FO B cells or both kinds of cells (Figure 3B), suggesting that components of the NF-κB pathway had been activated, undergone nuclear translocation, and affected the transcription of many genes.
Analyses of gene expression profiling of purified MZ and FO B cells from BCL10 Tg mice by qPCR. (A) Functional classification and enrichment analysis. The genes that distinguished MZ or FO B cells from WT and Tg mice were identified by t test at P < .05, then classified into functional categories by gene ontology. Enrichment of differentially expressed genes in each category for both types of B cells was examined by Fisher exact test. (B) List of known NF-κB target genes found to be differentially expressed genes with fold changes of greater than 1.5 in MZ or FO B cells of WT versus Tg mice.
Analyses of gene expression profiling of purified MZ and FO B cells from BCL10 Tg mice by qPCR. (A) Functional classification and enrichment analysis. The genes that distinguished MZ or FO B cells from WT and Tg mice were identified by t test at P < .05, then classified into functional categories by gene ontology. Enrichment of differentially expressed genes in each category for both types of B cells was examined by Fisher exact test. (B) List of known NF-κB target genes found to be differentially expressed genes with fold changes of greater than 1.5 in MZ or FO B cells of WT versus Tg mice.
Among the 25 NF-κB targets with altered expression were 12 with fold changes of greater than 1.5 that were altered in both subsets and commonly up- or down-regulated. Eight encoded Ig heavy chain isotypes and the λ light chain gene. Genes up-regulated in MZ and FO B cells of Tg/+ mice were Ikbkg, B2m, Tnfsf13b (which encodes BAFF, and Cd80, whereas Il1b was down-regulated in both. NF-κB targets uniquely up-regulated in MZ cells from Tg/+ mice were Traf5, Irf1, and Cxcl9 and for FO B cells included Nfkb1, Brca2, Myb, and Cd83. Conversely, target genes selectively down-regulated in the subsets were Ccnd2 and Fcer2a for MZ B cells and Snai1, Lef1, and Bcl3 for FO B cells (Figure 3B).
The 83 genes unrelated to NF-κB signaling that differed significantly between purified MZ and FO B cells from WT versus Tg/+ mice (supplemental Table 2) were also of interest. Twenty-one were up- or down-regulated in both MZ and FO B cells with the sum effect being biased toward reduced proliferation–enhanced expression of Ezh2 and Cdkn2c and reduced expression of Bcl9, Mll1, Cdk6, and Smad7. Interestingly, only 9 genes were selectively up-regulated (n = 3) or down-regulated (n = 6) in Tg/+ versus WT MZ B cells compared with 53 genes selectively up-regulated (n = 33) or down-regulated (n = 20) in Tg/+ versus WT FO B cells (supplemental Table 2). Thus, constitutive expression of BCL10 in B cells had a much more profound effect on gene expression in FO B cells than in MZ B cells but with the end effect being expansion of the MZ compartment.
Analysis of NF-κB signaling in cells of WT and Tg/+ mice
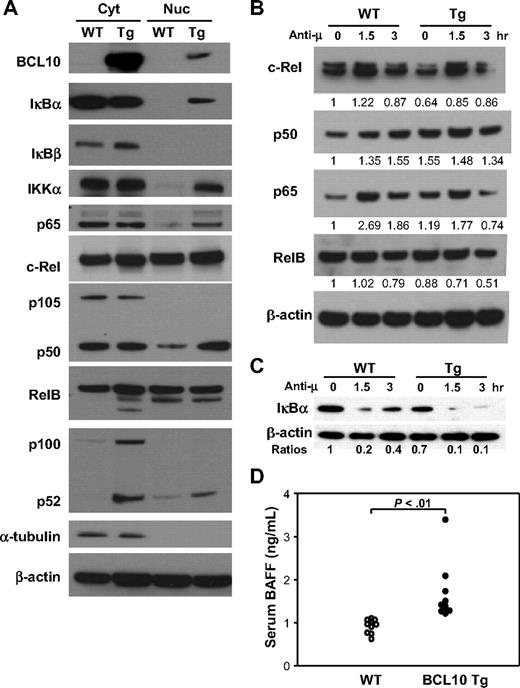
To evaluate the activity of NF-κB pathways in vivo, cytoplasmic and nuclear proteins from purified splenic B cells of Tg/+ and WT mice were probed by Western blotting for members of the NF-κB family belonging to both the canonical and noncanonical pathways (Figure 4A). Effects of constitutive BCL10 expression on the canonical pathway included decreased level of cytosolic IκBα associated with nuclear translocation of IκBα, IKKα, p50, and p65 and modestly increased levels of c-Rel in both the cytoplasm and nucleus. Effects on the noncanonical pathway were evidenced by increased levels of p100, the presence of high levels of p52 in the cytoplasm, and increased nuclear translocation of p52. Overexpression of BCL10 was thus associated with activation of both the canonical and the noncanonical NF-κB pathways. The extent to which both pathways are activated is reflected in the increased ratios of p50 and p52 in the nuclei of Tg/+ compared with WT B cells. Notably, low levels of BCL10 were regularly detected in nuclear extracts of B cells from Tg/+ mice (Figure 4A top panel).
Analyses of NF-κB signaling pathways in B cells of Tg mice. (A) Cytoplasmic (Cyt) and nuclear (Nuc) proteins were isolated from purified B cells of Tg and WT mice and analyzed by immunoblotting with Ab to the indicated proteins. The data are representative of 3 independent experiments. BCL10 was identified using a mAb that recognizes human but not mouse BCL10. (B) B cells purified from the spleens of Tg and WT littermates were stimulated with anti-IgM for the indicated times and harvested for preparation of nuclear extracts. Western blotting was used to assess expression levels of components of the canonical (c-Rel, p50, p65) and noncanonical (RelB) NF-κB signaling pathways. Levels of each protein were normalized to β-actin at time zero for WT cells and are indicated numerically. (C) Western blot analysis of IκBα and β-actin levels in splenic B cells stimulated by BCR ligation for the indicated times. The ratio of IκBα to β-actin levels is indicated for each time point normalized to a value of 1 the ratio for WT spleen cells at time zero. (D) Expression of BAFF in serum. The levels of BAFF in sera of 8- to 10-week-old WT and Tg mice were measured by ELISA. Each data point represents a single mouse.
Analyses of NF-κB signaling pathways in B cells of Tg mice. (A) Cytoplasmic (Cyt) and nuclear (Nuc) proteins were isolated from purified B cells of Tg and WT mice and analyzed by immunoblotting with Ab to the indicated proteins. The data are representative of 3 independent experiments. BCL10 was identified using a mAb that recognizes human but not mouse BCL10. (B) B cells purified from the spleens of Tg and WT littermates were stimulated with anti-IgM for the indicated times and harvested for preparation of nuclear extracts. Western blotting was used to assess expression levels of components of the canonical (c-Rel, p50, p65) and noncanonical (RelB) NF-κB signaling pathways. Levels of each protein were normalized to β-actin at time zero for WT cells and are indicated numerically. (C) Western blot analysis of IκBα and β-actin levels in splenic B cells stimulated by BCR ligation for the indicated times. The ratio of IκBα to β-actin levels is indicated for each time point normalized to a value of 1 the ratio for WT spleen cells at time zero. (D) Expression of BAFF in serum. The levels of BAFF in sera of 8- to 10-week-old WT and Tg mice were measured by ELISA. Each data point represents a single mouse.
Prior studies showing that BCR-induced activation of NF-κB depends on BCL10 prompted us to examine the NF-κB responses of B cells from WT and Tg/+ mice to this stimulus.2,3 Protein extracts from cells harvested 1.5 and 3 hours after stimulation were analyzed on Western blots for expression of NF-κB components. The data showed that canonical NF-κB signaling in Tg/+ B cells remained responsive to BCR stimulation, although the response appeared somewhat blunted compared with WT B cells (Figure 4B). This may reflect the fact that NF-κB is constitutively activated in BCL10-overexpressing cells, even in the absence of exogenous stimuli (Figure 4A). BCL10 overexpression also appears to activate the noncanonical NF-κB pathway (Figure 4A); however, RelB, as part of this pathway, was not substantively changed in Tg/+B cells compared with WT B cells (Figure 4B) nor were the levels of p100 and p52 differentially altered after BCR engagement (data not shown). This suggests that in Tg/+ B cells, baseline alterations in expression of noncanonical pathway components and the activation of noncanonical NF-κB signaling (Figure 4A) may occur independently of BCR-associated mechanisms.
Activation of the canonical NF-κB pathway results in phosphorylation of IκB proteins by activated IKK, inducing IκB ubiquitination and proteasomal degradation. To examine the status of this pathway, we activated purified splenic B cells of WT and Tg/+ mice by BCR ligation and tested for levels of IκBα expression 1.5 and 3 hours later. The results (Figure 4C) showed that IκBα levels were lower in resting B cells of Tg/+ mice than in WT mice and that activation resulted in greater and longer-lasting reductions in the levels of the protein. Thus, the canonical NF-κB pathway in Tg/+ B cells is constitutively active but remains responsive to stimulation through the BCR.
Constitutive activation of the noncanonical NF-κB pathway is of additional interest in view of the approximate 4-fold up-regulation of transcripts for Tnfsf13b, the gene encoding BAFF, in both MZ and FO B cells of Tg/+ mice (Figure 3B). BAFF activates NF-κB through both the canonical and noncanonical pathways, promoting B-cell survival, enlargement of the MZ B-cell pool, and retention of B cells in the MZ.25 High-level BAFF expression and activation of NF-κB have also been associated with several classes of non-Hodgkin lymphoma, including MALT lymphomas.26 To determine whether increased levels of Tnfsf13b transcripts correlated with elevated protein, we tested sera from Tg/+ and WT mice for levels of BAFF by ELISA. BAFF levels were significantly higher in sera of Tg/+ mice (P < .01; Figure 4D). This suggests that BCL10-induced up-regulation of BAFF could generate an autocrine loop, with enhanced survival and expansion of B cells due at least in part to the stimulation of both NF-κB pathways.
Overexpression of BCL10 alters humoral immune responses
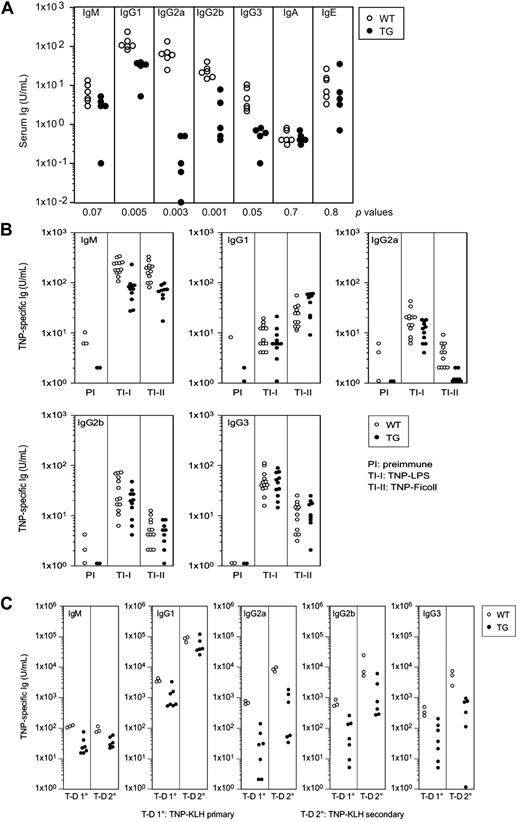
Quantitative studies showed that levels of IgM in sera of untreated Tg/+ mice were approximately half that in WT animals, whereas levels of all switched IgG classes were markedly lower. The serum levels of IgA and IgE were very similar for mice of both genotypes (Figure 5A).
Responses of Tg and WT mice to antigenic challenges. (A) Ig levels in sera of WT and Tg mice. Levels of serum Ig subclasses of mice 5 to 9 months of age (both sexes) were measured by ELISA. Data points indicate individual mice. Statistical (P) values assessing the differences in Ig levels between the 2 groups of mice are indicated at the bottom. (B) Levels of TNP-specific Ab among different Ig classes in sera of preimmune (PI) mice and mice immunized with the indicated TI-I or TI-II Ag as measured by ELISA. ○ indicate WT mice; ●, Tg/+ mice. (C) Levels of TNP-specific Ab among different Ig classes in sera of mice after primary (10) and secondary (20) immunizations with the TD Ag, TNP-KLH, as measured by ELISA. ○ indicate WT mice; ●, Tg/+ mice.
Responses of Tg and WT mice to antigenic challenges. (A) Ig levels in sera of WT and Tg mice. Levels of serum Ig subclasses of mice 5 to 9 months of age (both sexes) were measured by ELISA. Data points indicate individual mice. Statistical (P) values assessing the differences in Ig levels between the 2 groups of mice are indicated at the bottom. (B) Levels of TNP-specific Ab among different Ig classes in sera of preimmune (PI) mice and mice immunized with the indicated TI-I or TI-II Ag as measured by ELISA. ○ indicate WT mice; ●, Tg/+ mice. (C) Levels of TNP-specific Ab among different Ig classes in sera of mice after primary (10) and secondary (20) immunizations with the TD Ag, TNP-KLH, as measured by ELISA. ○ indicate WT mice; ●, Tg/+ mice.
To determine whether reduced serum Ig levels in Tg/+ mice reflected B-cell–intrinsic abnormalities, we analyzed isotype-specific, Ag-specific responses of mice immunized with the type I T-independent Ag (TI-I), TNP-LPS, and the type II TI Ag (TI-II), TNP-Ficoll. Tg/+ mice immunized with either Ag produced approximately 5-fold less TNP-specific IgM than WT mice (Figure 5B). Aside from the IgG2a response of Tg/+ mice, the class-switched Ag-specific responses of WT and Tg/+ mice to both Ags were generally similar. In Tg/+ and WT mice immunized with TNP-Ficoll, serum levels of IgG1, IgG2b, and IgG3 anti-TNP Ab were basically comparable, whereas serum levels of IgG2a Ag-specific Ab were substantially diminished for Tg/+ mice. Thus, except for IgM, the responses of Tg/+ mice to TI-I and TI-II Ags were largely similar to those of WT mice, and there was no significant defect in class switch recombination. Classically, Ab responses to TI-II Ags are generated by short-lived plasma cells that develop in extrafollicular sites from MZ or other B cells and can undergo class switching in the absence of T cells or germinal centers (GCs)27,28 ; however, some B-cell responses to T-dependent (TD) Ag have similar features.29,30 Thus, preservation of the MZ compartment in Tg/+ mice may have contributed to the relative normality of these responses.
These data suggest that the reduced Ig levels in the sera of unimmunized Tg/+ mice were not indicative of a B-cell–intrinsic defect but might be caused by impaired responses to T-cell help or defects in GC formation. To test these possibilities, we first examined primary Ab responses to the TD Ag, TNP-KLH in complete Freund adjuvant followed by a secondary response to Ag in incomplete Freund adjuvant. Compared with WT mice, the primary and secondary responses of Tg/+ mice were characterized by substantially lower levels of IgM and class-switched Ag-specific Ab (Figure 5C). We further examined this issue by analyzing spleen cells from mice immunized with TNP-KLH in alum 2 weeks previously for the frequencies of GC B cells that coexpress PNA and GL7 (supplemental Figure 2A). We found that the frequency of GC B cells in spleens of WT mice was nearly 4-fold higher than for Tg/+ mice. Furthermore IHC analyses of spleens stained with Ab to BCL6 showed that GCs in spleens of Tg/+ mice were smaller and reduced in number (supplemental Figure 2B). We conclude that B cells with constitutively activated BCL10 are impaired in their ability to respond to T-dependent Ags due, at least in part, to impaired GC formation.
Development of lymphomas in Tg/+, Tg/Tg, and WT mice
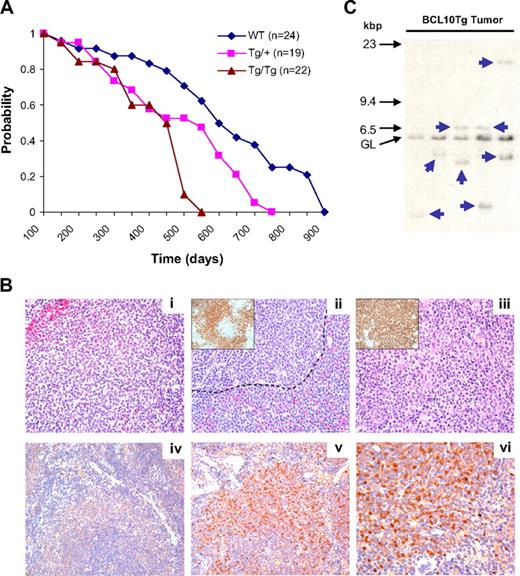
To determine whether BCL10 Tg mice develop lymphomas, groups of WT, Tg/+, and Tg/Tg mice were followed over a period of more than 900 days (Figure 6A). The average survival was 603 days for WT mice, 519 days for Tg/+ mice, and 429 days for Tg/Tg mice, indicating a Tg dose-dependent effect on survival. Necropsies were performed on 17 WT, 14 Tg/+, and 10 Tg/Tg mice that became moribund between 139 and 558 days of age (mean, 258 days). Among WT mice, there were 2 low-grade MZ lymphomas (MZLs), 1 early MZL, and 1 follicular lymphoma. For Tg/+ cases, there were 3 low-grade MZLs, 1 early MZL, and 1 case diagnosed with MZL and a centroblastic lymphoma in the mesenteric LN that did not represent a progression of MZL from low to high grade as described for some MZLs in NFS.V+ mice.19 Finally, we diagnosed 5 low-grade MZLs and 1 early MZL among the Tg/Tg mice. These findings suggest that FVB mice may be intrinsically predisposed to develop MZL, but that this predisposition is enhanced by constitutive expression of BCL10.
Survival and development of lymphomas in WT, Tg/+, and Tg/Tg mice. (A) Kaplan-Meier survival plot of overall survival of mice of the 3 genotypes. There is a statistically significant difference between survival curves (P = .041). Some mice were examined by necropsy when moribund. (B) Histologic and IHC features of spleens from Tg mice. (i) Low-grade splenic MZL showing enlarged MZ with atypical cells expanding into the red pulp (top left). Small follicular lymphocytes are at bottom right. (ii) Higher-grade MZL with centroblast-like cells both outside the marginal sinus (----) and in the follicle. (Inset) Same spleen stained with Ab to PAX5. (iii) High-grade MZL with the spleen almost totally occupied by cells with uniform features of centroblasts. (Inset) Same spleen stained with Ab to B220 emphasizes the almost total effacement of normal architecture by the aberrant B cells. (iv) Non-Tg spleen stained with Ab to human BCL10. (v) BCL10 Tg spleen with expanded MZ stained with Ab to human BCL10. (vi) Same spleen as in panel Bv, showing nuclear and cytoplasmic localization of BCL10. Histologic images were viewed with an Olympus Ax70 microscope (40×/0.75 oil objective lens) and photographed with an Olympus DP71 camera; DP controller software (Version 3.3.1.292) was used for image acquisition. (C) Southern blot hybridization analyses of Ig heavy chain gene organization in DNA prepared from spleens of 5 Tg/+ mice diagnosed with MZL. Locations of size markers and the germline (GL) band are shown on the left.
Survival and development of lymphomas in WT, Tg/+, and Tg/Tg mice. (A) Kaplan-Meier survival plot of overall survival of mice of the 3 genotypes. There is a statistically significant difference between survival curves (P = .041). Some mice were examined by necropsy when moribund. (B) Histologic and IHC features of spleens from Tg mice. (i) Low-grade splenic MZL showing enlarged MZ with atypical cells expanding into the red pulp (top left). Small follicular lymphocytes are at bottom right. (ii) Higher-grade MZL with centroblast-like cells both outside the marginal sinus (----) and in the follicle. (Inset) Same spleen stained with Ab to PAX5. (iii) High-grade MZL with the spleen almost totally occupied by cells with uniform features of centroblasts. (Inset) Same spleen stained with Ab to B220 emphasizes the almost total effacement of normal architecture by the aberrant B cells. (iv) Non-Tg spleen stained with Ab to human BCL10. (v) BCL10 Tg spleen with expanded MZ stained with Ab to human BCL10. (vi) Same spleen as in panel Bv, showing nuclear and cytoplasmic localization of BCL10. Histologic images were viewed with an Olympus Ax70 microscope (40×/0.75 oil objective lens) and photographed with an Olympus DP71 camera; DP controller software (Version 3.3.1.292) was used for image acquisition. (C) Southern blot hybridization analyses of Ig heavy chain gene organization in DNA prepared from spleens of 5 Tg/+ mice diagnosed with MZL. Locations of size markers and the germline (GL) band are shown on the left.
Histologically, MZLs at the earliest stage exhibited fingerlike extensions into the red pulp and compressed the white pulp (Figure 6B). MZ B cells at this stage were larger and more pleiomorphic than those in spleens of younger mice with enlarged MZs. In addition, some cells exhibited features of centroblasts with 2 or more small dense nucleoli at the nuclear membrane. In more advanced cases, a more uniform population of cells localized to both sides of the marginal sinus and penetrated the red pulp (Figure 6B); in such instances, a high proportion of the PAX5+ cells (Figure 6Bii inset) resembled centroblasts. In the most advanced cases, all that remained of the white pulp was a few small lymphocytes admixed with hemosiderin-laden macrophages, and the red pulp was reduced to thin bands surrounding the follicles (Figure 6Biii). This disease progression and the cytologic features of the B220+ cells that occupied almost the entire spleen (Figure 6Biii inset) were fully consistent with the diagnosis of splenic MZL.18,19
Sections of spleens of a WT mouse stained with a species-specific Ab to human BCL10 showed minimal background staining (Figure 6Biv); by contrast, staining of sections from the spleen of a Tg/+ mouse (Figure 6Bv-vi) showed intensely stained cells localized to the MZ while sparing the red pulp and residual white pulp (Figure 6Bv). Under higher power, the staining for BCL10 was both nuclear and cytoplasmic (Figure 6Bvi) rather than the cytoplasm-only pattern observed in normal, nontransformed BCL10-expressing B cells.1,14 We conclude that constitutive expression of BCL10 in B cells is insufficient for rapid development of highly penetrant lymphoma, but that, by histologic criteria, the proportion of mice that developed MZL was increased.
To determine whether the cases diagnosed histologically as lymphomas were clonal, DNA prepared from spleens of affected mice was examined by Southern blot hybridization for the organization of the Ig heavy chain locus (Figure 5C). The results of these studies showed that DNA from each of the 5 cases diagnosed as MZL had 1 or 2 distinct nongermline bands of various intensity, indicated by arrows, diagnostic of the presence of a clonal B-cell population. There was no substantial reduction in the intensity of the germline band in any sample, indicating that the clonal population comprised a subset of total spleen cells. Parallel studies of DNA prepared from spleen of Tg mice 3 to 6 months of age showed hybridization patterns consistent with the presence of polyclonal populations of B cells in most instances but 1 spleen with a clearly rearranged band (data not shown). These results indicate that constitutive expression of BCL10 in B cells results in the early preferential expansion of a polyclonal MZ B-cell population followed by the development of monoclonal MZ B-cell lymphomas.
Discussion
Characterizations of Bcl10−/− mice suggested that in MALT lymphomas bearing t(1;14)(p22;q32) translocations deregulated BCL10 contributes to the transformation and Ag-independent tumor progression by activating the prosurvival NF-κB pathway.2,3 Our studies of BCL10 Tg/+ mice provide the first direct in vivo evidence that overexpression of BCL10 in B cells results in multiple abnormalities in B-cell development and function and, eventually, transformation. Our data confirm that many of these abnormalities can be ascribed to constitutive activation of the canonical NF-κB pathway; however, we find the noncanonical pathway is also activated, possibly as part of an autocrine loop driven by BAFF. Constitutive activation of both NF-κB pathways resulted in altered expression of several genes involved in apoptosis, transcription, cell-cycle regulation, and signal transduction. Finally, we found BCL10 translocated to nuclei of B cells from Tg/+ mice, a feature of MALT lymphomas with either t(1;14) or t(11;18) translocations.1,14,31,32 The fact that not all Tg/+ mice developed lymphomas shows that aberrant expression of BCL10 is insufficient to drive lymphomagenesis, indicating that additional genetic events are required for full transformation.
In normal B cells, BCR ligation activates the CARMA1 signalosome, leading to activation of the IKK complex, degradation of IκB proteins, and release of NF-κB to the nucleus.4 Signalosome activation also stimulates rapid turnover of components of the complex, which could be responsible for signal termination. Indeed, K48-linked ubiquitination of BCL10 is associated with rapid, marked reductions in protein levels after Ag receptor ligation.1,14,33 Overexpression of BCL10 in B cells clearly changed the dynamics and magnitude of this signaling process, as indicated by constitutive activation of the canonical and noncanonical NF-κB pathways and significantly altered expression of many direct target genes of NF-κB. In addition, this stimulus led to expansion of the MZ B-cell compartment.
Many of the functional changes exhibited by B cells of Tg/+ mice suggest that BCL10 modulates the strength of signals initiated by BCR engagement. BCR signal strength is a critical determinant of the segregation and persistence of mature B cells in different pools, with the strongest signals driving B1 selection, weaker signals promoting MZ B development, and FO B cells requiring little or no signal for follicular localization.34-37 Basal BCR signaling mediated by formation of oligomeric BCR complexes in the absence of Ag ligation may be sufficient for FO B-cell survival.38 BCL10-induced amplification of BCR signal strength could thus result in biased development of MZ over FO B cells. The striking reduction in size of the peritoneal B1a B-cell compartment in Tg/+ mice suggests that heightened BCR signal strength during fetal B1a cell development might induce apoptosis, in analogy to the response of immature BM B cells to cell-bound self-Ags. This possibility is strengthened by the observation that a significant proportion of Tg/+ FO cells that normally would proliferate in response to BCR ligation instead underwent apoptosis. B1b B cells, functionally similar to B1a cells, were not depleted, but their origins from distinct progenitors may underlie differing responses to enhanced BCR signaling.34
Like BCL10 Tg mice, API2-MALT1 Tg mice exhibit impaired early B-cell development, enlarged MZs, and a reduced FO B-cell compartment. However, CD23 expression on API2-MALT1 Tg splenic B cells was normal. In addition, BM pre-B cells did not respond to IL-7 in vitro, whereas cultured BCL10 Tg/+ BM B-cell precursors proliferate normally to IL-7 (data not shown).39 These discrepancies may relate to the fact that BCL10 mediates BCR-triggered activation of both p65 (RelA) and c-Rel, whereas MALT1 appears to selectively activate c-Rel.40 Thus, BCL10 appears to have functions at least partially distinct from MALT1 in regulating B-cell differentiation, survival, and proliferation.
Purified Tg/+ B cells had increased levels of Tnfsf13b transcripts and expressed BAFF on the cell surface, and sera from these mice contained elevated levels of BAFF. These findings are consistent with studies showing that activated mouse B cells can produce BAFF transcripts and protein, and that expression is higher in M B cells than in FO B cells.41 BAFF can activate both the canonical and noncanonical NF-κB pathways to promote the survival and expansion of MZ B cells.25,42 Activation of BAFF by constitutive BCL10 could thus form a positive feedback loop as a contribution to MALT lymphomagenesis. The fact that anti-BAFF Ab in spleen cell cultures did not influence survival does not exclude the postulated activity of an autocrine loop because systemic effects of BAFF on B cells in vivo may not be well replicated with cultured B cells in vitro. Studies of mice treated with TACI-Ig might provide an approach to further explore this issue.
MALT lymphomas often occur in settings of chronic inflammation associated with autoimmune disorders or infections, states in which BAFF-expressing cells other than B cells are activated. This could promote survival and expansion of self-reactive B lymphocytes in normally “forbidden” FO and MZ niches,42 providing a chronically activated cell population susceptible to genetic errors. This concept is supported by studies showing significant elevations of BAFF levels in sera of patients with systemic lupus erythematosus, rheumatoid arthritis, and Sjögren syndrome,43-45 diseases associated with significantly increased risks of MALT lymphoma development. In addition, expression of BAFF and its receptors has recently been associated with MZ and other types of non-Hodgkin lymphoma.26,46,47
Aberrant nuclear expression of BCL10 is found in a significant proportion of MALT lymphomas but always in association with the cytoplasmic expression seen in normal B cells.1,14 The association of nuclear BCL10 with the API2-MALT1 translocation in human MALT lymphoma is somewhat paradoxical,1,32 because MALT1 normally promotes nuclear export of BCL10.48 Nuclear BCL10 is also seen in MALT lymphomas with t(1:14) translocations associated with constitutive activation of BCL10.49 The fact that nuclear BCL10 is a feature of B cells of BCL10 Tg/+ but not of API2-MALT1 Tg mice may contribute to the differential susceptibility of the 2 strains to lymphoma.39 Possibly, the secondary genetic errors that lead to full transformation do so in part by blocking nuclear export of BCL10 by MALT1 or promoting BCL10 nuclear translocation or both.
A subset of Tg/+ mice developed splenic MZL. API2-MALT1 Tg mice treated with Freund complete adjuvant developed MZL-like changes, but these resolved by 12 weeks after inoculation.50 The appearance of clonal disease in BCL10 Tg/+ mice may provide novel opportunities for identifying the contributions of genetic and environmental factors to the pathogenesis of BCL10-driven MALT lymphomas.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Torgny N. Fredrickson for helpful discussions and histologic diagnoses, Alfonso Macias for colony management, and Brenda Rae Marshall for editorial assistance.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases and by National Cancer Institute (NCI; grant R01 CA87064, S.W.M.), Cancer Center (CORE; grant CA21765), and the American Lebanese Syrian Associated Charities (ALSAC), St Jude Children's Research Hospital.
National Institutes of Health
Authorship
Contribution: Z.L., H.W., and L.X., designed and performed research, analyzed data, and wrote the paper; D.-M.S., D.R., W.X., C.-F.Q., M.Y.S., C.J.O., E.T., J.E.R., X.C., and Q.Z. performed research and analyzed data; and H.C.M. and S.W.M. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Quangeng Zhang, MD, Department of Immunology, Capital Medical University, Rm 1121, Second Teaching Bldg, #10 Xi-Tou-Tiao, You-An-Men, Beijing, P.R. China, 100069; e-mail: zhangqg@ccmu.edu.cn; Herbert C. Morse III, MD, Laboratory of Immunopathology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 5640 Fishers Ln, Rm 1421, Rockville, MD 20852; e-mail: hmorse@niaid.nih.gov; or Stephan W. Morris, MD, Department of Pathology, St. Jude Children's Research Hospital, Rm D4026, Mail stop #343, 262 Danny Thomas Pl, Memphis, TN 38105-3678; e-mail: steve.morris@stjude.org.
References
Author notes
Z.L., H.W., and L.X. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal