Abstract
The autosomal recessive immunodeficiencies Griscelli syndrome type 2 (GS2) and familial hemophagocytic lymphohistiocytosis type 3 (FHL3) are associated with loss-of-function mutations in RAB27A (encoding Rab27a) and UNC13D (encoding Munc13-4). Munc13-4 deficiency abrogates NK-cell release of perforin-containing lytic granules induced by signals for natural and antibody-dependent cellular cytotoxicity. We demonstrate here that these signals fail to induce degranulation in resting NK cells from Rab27a-deficient patients. In resting NK cells from healthy subjects, endogenous Rab27a and Munc13-4 do not colocalize extensively with perforin. However, phorbol 12-myristate 13-acetate and ionomycin stimulation or conjugation to susceptible target cells induced myosin-dependent colocalization of Rab27a and Munc13-4 with perforin. Unexpectedly, individual engagement of receptors leukocyte functional antigen-1, NKG2D, or 2B4 induced colocalization of Rab27a, but not Munc13-4, with perforin. Conversely, engagement of antibody-dependent cellular cytotoxicity receptor CD16 induced colocalization of Munc13-4, but not Rab27a, with perforin. Furthermore, colocalization of Munc13-4 with perforin was Rab27a-dependent. In conclusion, Rab27a or Munc13-4 recruitment to lytic granules is preferentially regulated by different receptor signals, demonstrating that individual target cell ligands regulate discrete molecular events for lytic granule maturation. The data suggest Rab27a facilitates degranulation at an early step yet highlight a reciprocal relationship between Munc13-4 and Rab27a for degranulation.
Introduction
Hemophagocytic syndromes are severe hyperinflammatory conditions and include a group of rare immune disorders with both familial and acquired forms.1-3 Typically, familial hemophagocytic syndromes entail autosomal recessive inheritance, affect infants, and are fatal unless treated by chemoimmunotherapy and subsequent hematopoietic stem cell transplantation (HSCT).4 Hallmarks of these immune disorders are hypercytokinemia and impairment of lymphocyte cytotoxicity.5,6 The diagnostic criteria for hemophagocytic lymphohistiocytosis (HLH) include prolonged fever, splenomegaly, cytopenia, hypertriglyceridemia/hypofibrinogenemia, hyperferritinemia, decreased or absent lymphocyte cytotoxicity, elevated soluble CD25 levels, and hemophagocytosis.4
For familial hemophagocytic lymphohistiocytosis (FHL), linkage analysis has so far identified 4 loci in the genomic regions 9q21.1-22 (FHL1, Mendelian Inheritance in Man [MIM] 267700), 10q21-22 (FHL2, MIM 603553), 17q25 (FHL3, MIM 608898), and 6q24 (FHL4, MIM 603552).7-10 Whereas no gene accountable for the disease at the 9q21.1-22 locus has been identified as yet, loss-of-function mutations in the genes for perforin (PRF1), Munc13-4 (UNC13D), and syntaxin 11 (STX11) account for FHL2, FHL3, and FHL4, respectively.8-10 Other syndromes, additionally characterized by partial albinism, are also associated with HLH.11 Griscelli syndrome type 2 (GS2, MIM 607624) is linked to genomic region 15q15-21 and caused by mutations in the gene for Rab27a (RAB27A).12 Likewise, Chediak-Higashi syndrome (MIM 214500) linked to 1q42.1-42.2 and Hermansky-Pudlak syndrome type 2 (MIM 608233) linked to 5q14.1 are caused by mutations in the genes encoding Lyst (LYST) and adaptor protein 3 β-subunit (ADTB3A), respectively.13,14
The identification of mutations in PRF1, UNC13D, STX11, RAB27A, LYST, and ADTB3A as causative of hemophagocytic syndromes combined with studies of the biologic function of their protein products have made a compelling link between HLH and impaired lymphocyte cytotoxicity.15,16 The perforin gene is transcribed by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells. Such cytotoxic lymphocytes store perforin in secretory lysosomes, specialized granules that mediate target cell killing. On degranulation, perforin facilitates granzyme-mediated apoptosis of target cells.17,18 The protein products of genes associated with hemophagocytic syndromes are involved in biogenesis, trafficking, and regulation of lytic granule release, providing mechanistic insight into cytotoxic lymphocyte effector function.9,12,19-21
Impaired NK-cell cytotoxicity is a diagnostic criterion for HLH.4 NK-cell cytotoxicity unfolds in discrete activation events and requires the cooperation of receptors that provide qualitatively different signals.22,23 To gain further insight into the general mechanisms of lymphocyte cytotoxicity, studies focusing on NK-cell functional deficits in patients with HLH offer several advantages. As opposed to CTLs, whose antigen receptor specificities are clonally distributed, NK cells can be activated through several increasingly well-characterized, uniformly expressed activating receptors.24 Moreover, although few CTL express perforin and stimulation is required to induce biogenesis of lytic granules, all NK cells contain abundant intracellular perforin.19 Accordingly, freshly isolated NK cells are well suited for studying the mechanisms of cytotoxicity by primary lymphocytes.
The steps leading to fusion of lytic granules with the plasma membrane represent a conundrum in cytotoxic lymphocyte biology. Rab27a is a member of the Rab GTPase family that regulates discrete steps of vesicle trafficking. In patients with RAB27A mutations, granule docking at the plasma membrane and subsequent degranulation are impaired in CTL.12,25,26 Remarkably, conclusions from a study of a single GS2 patient have indicated that, despite the dependence of Rab27a in NK cell–mediated natural cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC) was intact in Rab27a-deficient NK cells.27 Thus, it is unclear whether the molecular pathways for granule release are shared in T-cell receptor–mediated, Fc receptor–mediated, and natural cytotoxicity. Binding assays performed in vitro have identified Munc13-4 as an effector of GTP-bound Rab27a.28,29 Munc13-4, belonging to a family of proteins that regulate membrane fusion, is required for docking and fusion of perforin-containing granules with the plasma membrane in CTL and NK cells.9,30,31 Studies of a mast cell line have provided evidence for localization of transfected and tagged Rab27a and Munc13-4 to the limiting membrane of secretory lysosomes, as determined by electron and confocal microscopy.29 In platelets, however, endogenous Rab27a is associated with dense granules, whereas Munc13-4 is divided between soluble and nondense granule membrane fractions.28 A recent study by Menager et al,32 in which interleukin-2 (IL-2)–cultured CTLs were transfected with fluorescently tagged Munc13-4 and Rab27a, demonstrated that Munc13-4 initially mediates assembly of Rab11+ recycling endosomes with Rab27a+ late endosomes independently of Rab27a and thereafter primes granule fusion, possibly through Rab27a. These results imply that the process of lytic granule exocytosis is more complex than previously appreciated, necessitating studies of endogenous proteins in unmanipulated cytotoxic lymphocytes.
Here, we demonstrate that NK-cell degranulation is more adversely affected by loss-of-function mutations in Munc13-4 than those in Rab27a. Still, degranulation triggered by both natural cytotoxicity and Fc-receptor engagement was significantly impaired in NK cells from Rab27a-deficient patients. Remarkably, in resting NK cells from healthy donors, engagement of distinct NK-cell receptors by physiologic ligands preferentially recruited Rab27a or Munc13-4 to perforin-containing granules. The results support the notion of myosin-dependent lytic granule maturation before exocytosis and suggest that recruitment of Rab27a to perforin-containing granules represents an early step in NK cell–mediated cytotoxicity.
Methods
Patients and healthy control donors
This study was approved by the Regional Ethics Review Board in Stockholm (approval nos. 2006/228-31/3, 2006/229-31/3, 2006/230-31/3, and 2008/1689-32). Peripheral blood from children with suspected HLH was obtained with the informed consent of their parents in accordance with the Declaration of Helsinki. Genetic analysis was subsequently performed as described in the next paragraph. For controls, blood was obtained from healthy volunteers from the Karolinska University Hospital with the donors' informed consent.
Mutation analysis
Genomic DNA was isolated from peripheral blood by standard procedures. Primers were designed for amplification and direct DNA sequencing of each coding exon and flanking regions of RAB27A and UNC13D (primer sequences and sequencing conditions provided on request). All sequencing reactions were done using BigDye terminators Version 3.1 Cycle Sequencing Kit (Applied Biosystems). Sequencing reactions were run on an ABI 3730 (Applied Biosystems) and analyzed in SeqScape (Applied Biosystems).
Cells
Peripheral blood mononuclear cells (PBMCs) were separated from whole blood by density gradient centrifugation (Lymphoprep, Axis Shield). NK cells were isolated from PBMCs by negative selection (Miltenyi Biotec), maintained in complete medium (RPMI 1640 medium containing 2 mM l-glutamine and 10% fetal bovine serum [FBS]; all Invitrogen), and used within 2 days of isolation. These cells were determined by flow cytometry to be more than 98% pure CD56+CD3− NK cells. For IL-2 stimulation, cells were cultured for 72 hours in complete medium supplemented with 400 U/mL recombinant human IL-2 (Chiron). The human erythroleukemia cell line K562 and mouse mastocytoma cell line P815 (both ATCC) were maintained in complete medium. Drosophila Schneider 2 (S2) cells were maintained in Schneider medium with 2 mM l-glutamine and 10% FBS (all Invitrogen) and induced for expression of ligands as previously described.33,34
Antibodies
For flow cytometry, fluorochrome-conjugated mouse anti-CD3 (UHCT1), anti-CD8 (SK1), anti-CD56 (NCAM16.2), anti-CD107a (H4A3), and anti-perforin (δG9; all from BD Biosciences) monoclonal antibodies (mAbs) were used. Purified mouse anti-CD3 (UHCT1) and anti-CD16 (3G8) mAbs were used for redirected ADCC (both from BD Biosciences). For confocal microscopy, mouse anti-CD107a (H4A3; BD Biosciences), anti–α-tubulin (clone 236-10501; Invitrogen), and anti-perforin (δG9; BioLegend) mAbs were used. In addition, rabbit anti-Rab27a, anti–Munc13-4 polyclonal antibodies were used.28 Secondary antibodies were donkey anti–mouse and donkey anti–rabbit (both from Invitrogen).
Cytotoxicity assays
Standard 4-hour 51Cr-release assays were performed according to previously established protocols for clinical samples.35 Briefly, 4 × 10451Cr-labeled K562 target cells were incubated with peripheral blood effector cells in 200 μL of complete medium in 96-well V-bottom plates. Experiments were performed in triplicate, and effector-to-target cell ratios ranged from 50 to 3. The supernatants were measured for 51Cr release on a gamma-counter (Cobra Auto-Gamma; Packard). Lytic units at 25% target cell lysis were calculated.
Degranulation assays
For quantification of lytic granule exocytosis, 2 × 105 PBMCs were mixed with 2 × 105 target cells and supplemented with 2.5 μg/mL mAb, where indicated. Cells were incubated for 2 hours at 37°C. Thereafter, cells were spun down, cell pellets resuspended in PBS supplemented with 2% FBS and 2mM ethylenediaminetetraacetic acid, stained with anti-CD3, anti-CD8, anti-CD56, and anti-CD107a mAbs, followed by flow cytometric analysis (FACSCalibur; BD Biosciences). Data were analyzed with FlowJo software (TreeStar). Lymphocytes were gated on forward scatter/side scatter characteristics. For intracellular labeling, cells were surface-stained, fixed with 4% formaldehyde (Sigma-Aldrich) in PBS, permeabilized with PBS supplemented with 2% FBS, 2 mM ethylenediaminetetraacetic acid, and 0.5% saponin (Sigma-Aldrich), and labeled for intracellular markers, as previously described.19
Immunofluorescence and confocal microscopy
Purified NK cells alone, stimulated with 100 ng/mL phorbol 12-myristate 13-acetate (PMA; Calbiochem) and 0.5 mM ionomycin (Sigma-Aldrich), coincubated on glass slides with S2 or K562 cells, or with ligand-coated beads, for 20 minutes at 37°C (Erie Scientific Company). In some experiments, cells were pretreated with ML-9 (Calbiochem) for 20 minutes at indicated concentrations. For coating of beads, 5.5-μm protein A–coated beads (Bangs Laboratories) were washed twice in water, incubated with 1 μg/μL purified human IgG (Sigma-Aldrich), or recombinant human ICAM-1-Fc (a gift from E. Long, National Institutes of Health, Rockville, MD)36 for 2 hours at 4°C, then washed 3 times and resuspended in RPMI 1640 medium. After stimulation, cells were fixed with 4% paraformaldehyde in PBS, permeabilized with PBS supplemented with 0.5% saponin, and blocked in PBS containing 5% FBS, 0.1% BSA-c (Aurion), and 2% normal donkey serum (Jackson ImmunoResearch Laboratories). Slides were mounted using Prolong Gold with 4,6-diamidino-2-phenylindole (Invitrogen), after which images were acquired on a confocal microscope (DMIRE2; Leica) with a 63× glycerol objective, using Leica Confocal Software (Version 2.61), and analyzed using ImageJ software (Version 1.410; Research Service Branch, National Institutes of Health, Bethesda, MD), with the Pearson correlation coefficient, r, determined using the JACOP plugin.37 Images for figures were generated by reconstruction of multiple optical sections.
Statistics
Statistical analysis was performed using Prism software, Version 5 (GraphPad Software), as specified.
Results
Impaired NK-cell degranulation and cytotoxicity in GS2 patients
To compare the requirement for Rab27a and Munc13-4 in lymphocyte cytotoxicity, degranulation and target cell killing by PBMCs from GS2 and FHL3 patients were examined. Three GS2 patients (patients 1-3) who fulfilled the clinical criteria for HLH and had defined mutations in RAB27A were identified (Table 1). Patients 1 and 2 were identical twins carrying compound heterozygous mutations in RAB27A (c.239G → C and c.550C → T). Patient 3 was homozygous for a nucleotide substitution in RAB27A (c.240-2A → C). Both the RAB27A c.239G → C and c.240-2A → C are novel mutations, whereas c.550C → T has been described.12 These patients were compared with 4 FHL3 patients (patients 4-7) who fulfilled the clinical criteria for HLH (Table 1). Patient 4 was homozygous for a nucleotide substitution in UNC13D (c.1145G → A).19 Patient 5 carried compound heterozygous nucleotide substitutions in UNC13D (c.247C → T and novel c.754-2A → G).31 Patient 6 bore compound heterozygous mutations in UNC13D (c.1389 + 1G → A and novel c.2477_2480delTCAC).9 Patient 7 was homozygous for a nucleotide substitution (c.753 + 1G → T).9
Familial data, mutation data, and clinical findings in patients with biallelic RAB27A and UNC13D mutations
| . | RAB27A (GS2) . | UNC13D (FHL3) . | |||||
|---|---|---|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | |
| Origin | Sweden | Sweden | Pakistan | Pakistan | The Netherlands | Denmark | Poland |
| Consanguinity | No | No | Yes | Yes | No | No | No |
| Sex | Female | Female | Female | Male | Female | Female | Female |
| Mutation | c.239G→C c.550C→T | c.239G→C c.550C→T | c.240-2A→C (homozygous) | c.1145G→A (homozygous) | c.247C→T c.754-2A→G | c.1389+1G→A c.2477_2480delTCAC | c.753+1G→T (homozygous) |
| Protein alteration | Splice error R184X | Splice error R184X | Splice error | W382X | R83X splice error | Splice error L826QfsX20 | Splice error |
| Age at HLH diagnosis | 2 mo | 2 mo | 13 y | 2 mo | 1 mo | 2 mo | 7 mo |
| Fever (> 38°C) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Splenomegaly | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hepatomegaly | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Hemoglobin, g/L | 46 | 62 | 81 | 52 | 79 | — | 82 |
| Neutrophils, 109/L | 0.7 | 0.3 | 2.0 | 0.2 | 0.7 | — | 0.3 |
| Platelets, 109/L | 10 | 85 | 53 | 5 | 9 | — | 2 |
| Triglycerides, mmol/L | 3.6 | 3.3 | ND | 3.3 | 4.2 | — | 3.7 |
| Fibrinogen, g/L | ND | ND | 1.9 | 0.6 | ND | ND | 0.6 |
| Ferritin, μg/L | 2293 | 1596 | 940 | 19 914 | 12 160 | — | 594 |
| Hemophagocytosis | ND | ND | No | Yes (spleen) | No | Yes (BM) | Yes (BM) |
| sCD25, U/mL | ND | ND | ND | ND | 8350 | ND | ND |
| Decreased NK-cell activity | Borderline* | Yes | Borderline† | Yes | Yes | Yes | Yes |
| Neurologic disease‡ | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Treatment protocol | HLH-2004 | HLH-2004 | HLH-2004 | HLH-2004 | HLH-2004 | HLH 2004 | HLH-2004 |
| Remission at 2 mo | No | No | No | ND | No | Yes | Yes |
| HSCT | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Outcome | Alive | Alive | Alive | Dead | Alive | Dead | Dead |
| . | RAB27A (GS2) . | UNC13D (FHL3) . | |||||
|---|---|---|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | |
| Origin | Sweden | Sweden | Pakistan | Pakistan | The Netherlands | Denmark | Poland |
| Consanguinity | No | No | Yes | Yes | No | No | No |
| Sex | Female | Female | Female | Male | Female | Female | Female |
| Mutation | c.239G→C c.550C→T | c.239G→C c.550C→T | c.240-2A→C (homozygous) | c.1145G→A (homozygous) | c.247C→T c.754-2A→G | c.1389+1G→A c.2477_2480delTCAC | c.753+1G→T (homozygous) |
| Protein alteration | Splice error R184X | Splice error R184X | Splice error | W382X | R83X splice error | Splice error L826QfsX20 | Splice error |
| Age at HLH diagnosis | 2 mo | 2 mo | 13 y | 2 mo | 1 mo | 2 mo | 7 mo |
| Fever (> 38°C) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Splenomegaly | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hepatomegaly | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Hemoglobin, g/L | 46 | 62 | 81 | 52 | 79 | — | 82 |
| Neutrophils, 109/L | 0.7 | 0.3 | 2.0 | 0.2 | 0.7 | — | 0.3 |
| Platelets, 109/L | 10 | 85 | 53 | 5 | 9 | — | 2 |
| Triglycerides, mmol/L | 3.6 | 3.3 | ND | 3.3 | 4.2 | — | 3.7 |
| Fibrinogen, g/L | ND | ND | 1.9 | 0.6 | ND | ND | 0.6 |
| Ferritin, μg/L | 2293 | 1596 | 940 | 19 914 | 12 160 | — | 594 |
| Hemophagocytosis | ND | ND | No | Yes (spleen) | No | Yes (BM) | Yes (BM) |
| sCD25, U/mL | ND | ND | ND | ND | 8350 | ND | ND |
| Decreased NK-cell activity | Borderline* | Yes | Borderline† | Yes | Yes | Yes | Yes |
| Neurologic disease‡ | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Treatment protocol | HLH-2004 | HLH-2004 | HLH-2004 | HLH-2004 | HLH-2004 | HLH 2004 | HLH-2004 |
| Remission at 2 mo | No | No | No | ND | No | Yes | Yes |
| HSCT | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Outcome | Alive | Alive | Alive | Dead | Alive | Dead | Dead |
— indicates not applicable; ND, no data available; and BM, bone marrow.
A total of 22 lytic units (reference value > 10 lytic units).
A total of 11 lytic units (reference value > 10 lytic units).
Neurologic disease relates to the entire course of the disease.
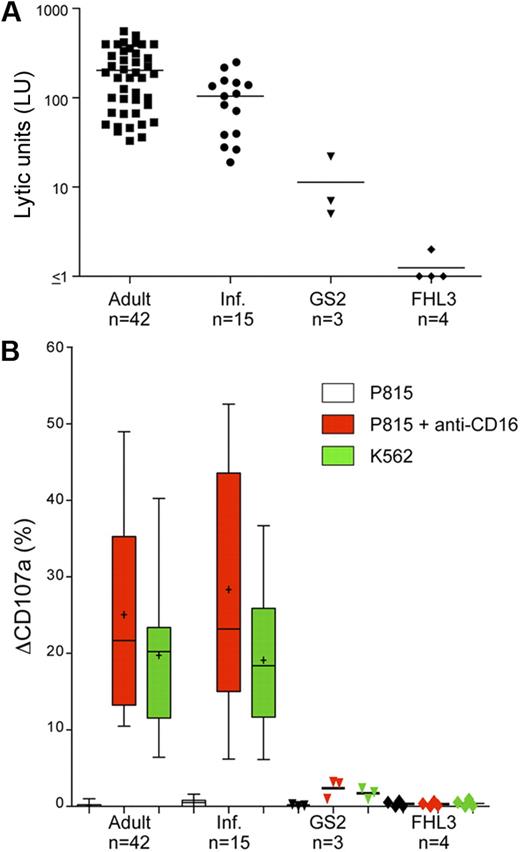
As expected, freshly isolated, unstimulated PBMCs from healthy adult and infant donors lysed NK cell–susceptible K562 cells in a 4-hour 51Cr-release assay (203.70 ± 144.70 lytic units at 25% target cell lysis [LU], n = 42 and 104.40 ± 70.78 LU; n = 15, respectively, [mean ± SD]; Figure 1A). PBMCs from healthy infant donors were less efficient at lysis of K562 cells relative to PBMCs from healthy adult controls (Figure 1A), perhaps because of a higher frequency of NK cells in adult peripheral blood, or qualitative differences between adult and infant NK cells.19 In comparison, freshly isolated unstimulated PBMCs from GS2 patients were severely impaired in their ability to lyse K562 cells (patient 1, 5 LU; patient 2, 22 LU; and patient 3, 7 LU; Figure 1A), and even less lysis was observed with PBMCs from FHL3 patients (patient 4, 1 LU; patient 5, 1 LU; patient 6, 1 LU; patient 7, 2 LU; Figure 1A). Perforin expression was detected in NK cells from all patients by flow cytometry (data not shown). Thus, PBMCs from GS2 patients exhibited significant impairment of target cell lysis yet relatively less impairment than PBMCs from FHL3 patients.
Impaired degranulation and cytotoxicity by resting NK cells from GS2 patients. (A) Resting PBMCs from healthy adult and infant donors plus GS2 and FHL3 patients were evaluated for cytotoxicity toward K562 cells in a 4-hour 51Cr-release assay. Lytic units (LU) at 25% target cell lysis were calculated from specific lysis values. Each point represents 1 person, with lines indicating mean values. (B) Resting PBMCs were incubated alone or with target cells as indicated for 2 hours at 37°C. Thereafter, cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. Lymphocytes were gated on forward/side scatter plots, followed by gating on CD3 versus CD56 plots. Induced CD107a surface expression on CD3−CD56+ NK cells after indicated stimulation was plotted. Plus symbols represent mean values; error bars, SD. Boxes represent 25th, 50th, and 75th percentiles. For patient groups, each point represents 1 person, with lines indicating mean values.
Impaired degranulation and cytotoxicity by resting NK cells from GS2 patients. (A) Resting PBMCs from healthy adult and infant donors plus GS2 and FHL3 patients were evaluated for cytotoxicity toward K562 cells in a 4-hour 51Cr-release assay. Lytic units (LU) at 25% target cell lysis were calculated from specific lysis values. Each point represents 1 person, with lines indicating mean values. (B) Resting PBMCs were incubated alone or with target cells as indicated for 2 hours at 37°C. Thereafter, cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. Lymphocytes were gated on forward/side scatter plots, followed by gating on CD3 versus CD56 plots. Induced CD107a surface expression on CD3−CD56+ NK cells after indicated stimulation was plotted. Plus symbols represent mean values; error bars, SD. Boxes represent 25th, 50th, and 75th percentiles. For patient groups, each point represents 1 person, with lines indicating mean values.
To assess degranulation, freshly isolated PBMCs were mixed with target cells and incubated for 2 hours at 37°C, followed by quantification of CD107a surface expression by flow cytometry.19,36 Stimulation was achieved by reverse ADCC after incubation of mouse FcR+ P815 cells with anti-CD16 mAbs, or by mixing with K562 cells. Lymphocytes were gated on forward-scatter/side-scatter characteristics and further identified according to expression of the lineage markers CD3 and CD56. In patients as well as controls, less than 1% of CD3−CD56+ NK cells were positive for surface CD107a when incubated without target cells. Moreover, P815 cells, which are not sensitive to NK-cell killing, did not induce NK-cell degranulation. However, after incubation with K562 cells, substantial degranulation was observed in NK cells from healthy adult and infant donors (19.12% ± 9.71%, n = 42; and 17.94% ± 9.84%; n = 15, respectively, [mean ± SD]; Figure 1B). In contrast, degranulation was impaired in GS2 patients, as assessed by CD107a surface expression on NK cells after incubation with K562 cells (patient 1, 1.8%; patient 2, 1.0%; and patient 3, 2.4%; Figure 1B). For comparison, degranulation was virtually absent in FHL3 patients (patient 4, 0.1%; patient 5, 0.9%; patient 6, 0.0%; patient 7, 0.5%; Figure 1B), as previously described.20,30,31 Similarly, anti-CD16 mAb stimulation induced CD107a surface expression on NK cells from healthy adult and infant donors (25.05% ± 12.51%; n = 42; and 28.36 ± 15.97%; n = 15, respectively), whereas degranulation was impaired or absent in the GS2 and FHL3 patients (patient 1, 3.0%; patient 2, 1.0%; and patient 3, 3.2%; patient 4, 0.4%; patient 5, 0.7%; patient 6, 0.0%; patient 7, 0.1%; Figure 1B). Notably, NK cells from all patients expressed normal levels of intracellular CD107a (data not shown). These data demonstrated that degranulation, induced either by receptors for natural cytotoxicity or by CD16 for ADCC, is impaired in GS2 patients. Still, in agreement with cytotoxicity data, NK cells from GS2 patients displayed less impairment of degranulation relative to FHL3 patients.
Previously, stimulation of PBMCs with IL-2 for 3 days partially restored degranulation and cytotoxicity in FHL4 patients.19 Of note, IL-2 stimulation did augment cytotoxicity and degranulation in GS2 patients to a greater extent than that in FHL3 patients (data not shown).
NK-cell activation induces colocalization of Rab27a and Munc13-4 with perforin
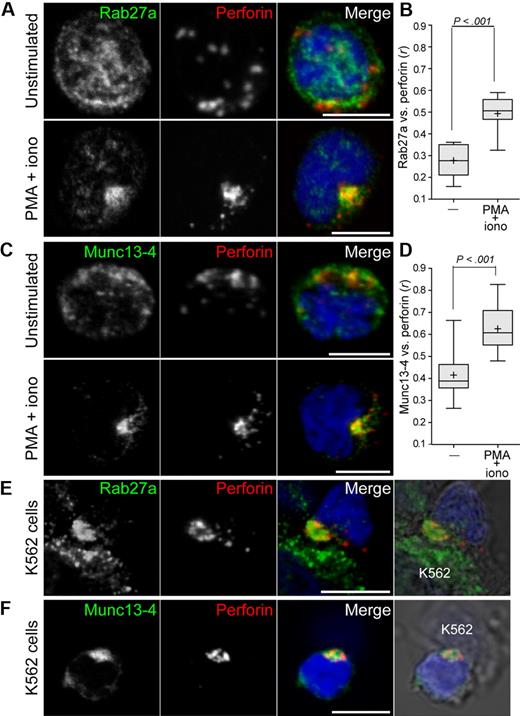
Although biochemical experiments have revealed that Munc13-4 can interact with GTP-bound Rab27a,28,29 it is still unclear how Rab27a and Munc13-4 facilitate lytic granule exocytosis. To gain insight into how these proteins regulate events preceding exocytosis, the localization of endogenous Rab27a and Munc13-4 was assessed in freshly isolated, resting NK cells from healthy donors (Figure 2). NK cells were fixed, permeabilized, and labeled with an anti-perforin mAb and either anti-Rab27a or anti-Munc13-4 polyclonal antibodies. Notably, both Rab27a and Munc13-4 displayed diffuse punctate labeling throughout the cells, which generally did not overlap with perforin (Figure 2A,C). A combination of the protein kinase C agonist PMA and the calcium ionophore ionomycin is frequently used to provide generic signals for lymphocyte activation. Stimulation of NK cells with PMA and ionomycin for 20 minutes at 37°C induced perforin clustering. This clustering was hypothesized to reflect lytic granule migration toward the microtubule organizing center, as recently described in CTL.38 Supporting this notion, after PMA and ionomycin stimulation, perforin was grouped around the bright spot of α-tubulin labeling at the center of the microtubule network (data not shown). Remarkably, PMA and ionomycin stimulation induced Rab27a clustering and extensive colocalization with perforin (Figure 2A). Colocalization between Rab27a and perforin was quantified as the Pearson coefficient r,37 and significance calculated using Student 2-tailed paired t test. Colocalization between Rab27a and perforin increased significantly on stimulation (0.28 ± 0.07 vs 0.50 ± 0.08; mean ± SD; Figure 2B). PMA and ionomycin stimulation also induced comparable Munc13-4 clustering and a significant increase of Munc13-4 colocalization with perforin (0.38 ± 0.07 vs 0.59 ± 0.08; Figure 2D). To determine whether susceptible target cells also induced colocalization of Rab27a and Munc13-4 with perforin, NK cells were incubated with K562 cells for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling. Induction of Rab27a or Munc13-4 colocalization with perforin was recapitulated in NK cells that had formed conjugates with K562 cells (Figure 2E-F). Thus, generic activation signals and susceptible target cells could induce colocalization of the effector proteins Rab27a and Munc13-4 to lytic granules in resting NK cells. These results corroborate findings in CTL, in which mAb-mediated T-cell receptor stimulation induced the coalescence of transfected Munc13-4 and Rab27a fusion proteins with perforin-containing granules.32
Induction of Rab27a and Munc13-4 colocalization with perforin on activation of resting NK cells. Resting NK cells were incubated alone, stimulated with PMA and ionomycin, or incubated with K562 cells, as indicated, for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling. Cells were labeled with anti-perforin mAb and polyclonal antibodies to Rab27a (A,E) or Munc13-4 (C,F). Confocal images of single, representative cells are shown, with scale bars representing 5 μm. Colocalization between perforin and Rab27a (B) or Munc13-4 (D) was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ and averaged for 10 to 15 cells from 10 individual donors. Plots represent cumulative data from 10 to 15 cells from each of 5 donors. Plus symbols represent mean values, and error bars represent SD. Boxes represent the 25th, 50th, and 75th percentiles. Two-tailed paired Student t tests were performed to assess significance.
Induction of Rab27a and Munc13-4 colocalization with perforin on activation of resting NK cells. Resting NK cells were incubated alone, stimulated with PMA and ionomycin, or incubated with K562 cells, as indicated, for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling. Cells were labeled with anti-perforin mAb and polyclonal antibodies to Rab27a (A,E) or Munc13-4 (C,F). Confocal images of single, representative cells are shown, with scale bars representing 5 μm. Colocalization between perforin and Rab27a (B) or Munc13-4 (D) was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ and averaged for 10 to 15 cells from 10 individual donors. Plots represent cumulative data from 10 to 15 cells from each of 5 donors. Plus symbols represent mean values, and error bars represent SD. Boxes represent the 25th, 50th, and 75th percentiles. Two-tailed paired Student t tests were performed to assess significance.
Recruitment of Rab27a and Munc13-4 to lytic granules is myosin-dependent
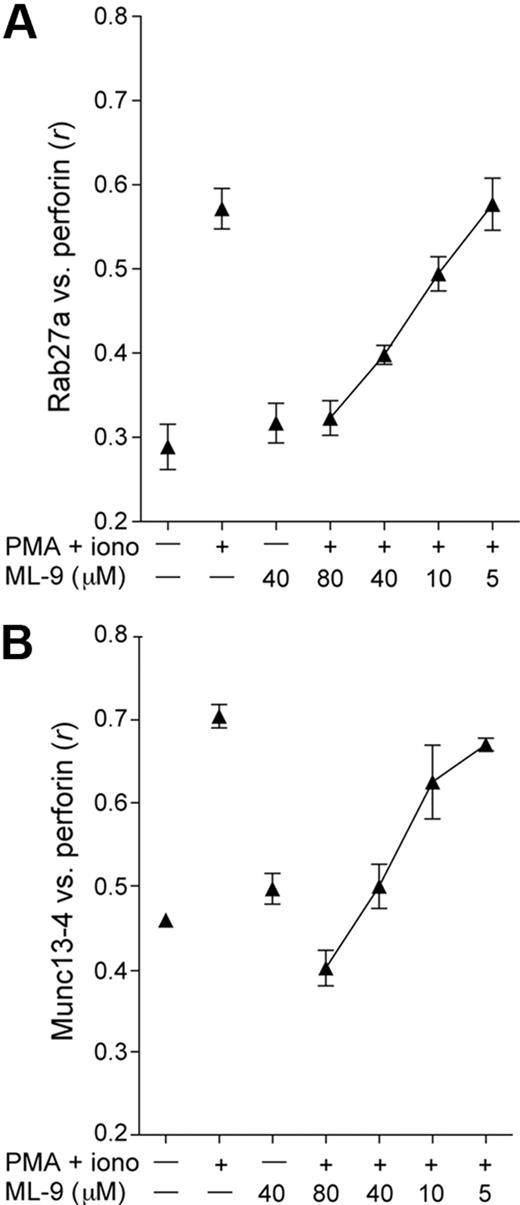
Rab27a and Munc13-4 may become associated with lytic granules through the fusion of hetereotypic organelles with perforin-containing secretory lysosomes, as described in CTL.32 Alternatively, effector proteins may be recruited to granules directly from the cytosol. Cytosolic pools of both Rab27a and Munc13-4 exist in platelets and could thus be available for direct recruitment to granules.28,39 To assess whether recruitment of Rab27a and Munc13-4 to granules is dependent on the actin motor protein myosin, ML-9, a pharmacologic inhibitor of myosin light chain kinase (MLCK) was used. MLCK can activate myosin IIA, which facilitates NK-cell exocytosis subsequent to lytic granule polarization to the immune synapse.40 ML-9 inhibited PMA and ionomycin induced colocalization of perforin with Rab27a or Munc13-4 in a dose-dependent manner (Figure 3). Moreover, ML-9 inhibited NK-cell degranulation (data not shown), as previously reported.40,41 Thus, recruitment of Rab27a and Munc13-4 to perforin granules was myosin-dependent. These data suggest that Rab27a and Munc13-4 association with granules is dependent on actin-based movement of proteins or subcellular compartments. This outcome is consistent with the hypothesis that granules fuse with other compartments before their release, as described for CTL.32
Rab27a and Munc13-4 recruitment to perforin granules is dependent on myosin function. Resting NK cells were treated with PMA and ionomycin alone or in combination with ML-9 at the indicated concentrations for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling. Colocalization of perforin with Rab27a (A) or Munc13-4 (B) was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ, with 10 to 15 cells per condition analyzed from each of 2 healthy donors. Mean values are plotted; error bars represent SD.
Rab27a and Munc13-4 recruitment to perforin granules is dependent on myosin function. Resting NK cells were treated with PMA and ionomycin alone or in combination with ML-9 at the indicated concentrations for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling. Colocalization of perforin with Rab27a (A) or Munc13-4 (B) was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ, with 10 to 15 cells per condition analyzed from each of 2 healthy donors. Mean values are plotted; error bars represent SD.
Recruitment of Munc13-4 to lytic granules is defective in Rab27a-deficient resting NK cells
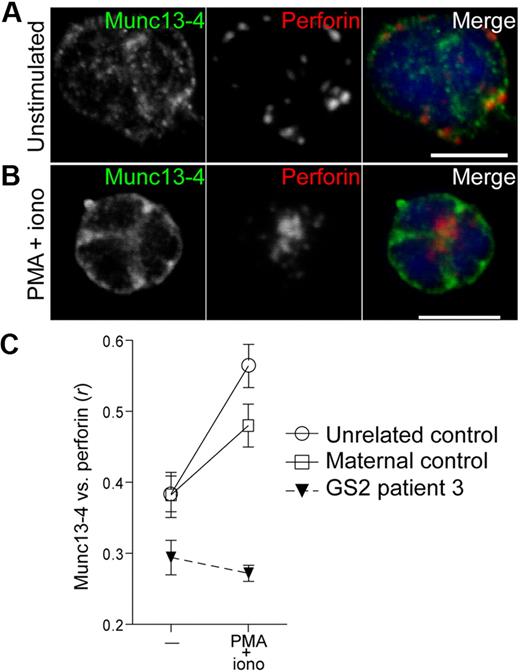
In CTL, Munc13-4 is required for induction of Rab27a colocalization with perforin.32 Likewise, in Munc13-4–deficient resting NK cells, Rab27a failed to colocalize with perforin on stimulation (data not shown). Conversely, resting NK cells from a GS2 patient were used to assess the requirement of Rab27a for Munc13-4 recruitment to perforin granules. In unstimulated Rab27a-deficient NK cells, Munc13-4 displayed a dispersed punctate labeling and minimal overlap with perforin, comparable with NK cells from healthy donors (Figure 4A). As in healthy controls, when Rab27a-deficient NK cells were stimulated with PMA and ionomycin, perforin granules clustered at one point in the cell. However, Munc13-4 did not polarize or colocalize with perforin but remained distributed throughout the cells, as opposed to its behavior in heterozygous maternal or healthy control cells (Figure 4B-C). Thus, Rab27a is required for recruitment of Munc13-4 to perforin granules. Together, the results suggest a reciprocal dependency for presence of Rab27a and Munc13-4 for perforin granule recruitment.
NK cells isolated from a GS2 patient are impaired in the ability to recruit Munc13-4 to polarized perforin granules in PMA/ionomycin-activated cells. Resting NK cells were isolated from a patient, a healthy heterozygous parent, and a healthy donor, then were incubated alone or stimulated with PMA and ionomycin for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling with anti-Rab27a polyclonal antibody and anti-perforin mAb. Confocal images of single, representative resting (A) and PMA/ionomycin-stimulated (B) Rab27a-deficient cells are shown, with scale bars representing 5 μm. (C) Colocalization of perforin with Munc13-4 was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ. Ten to 15 cells per condition were analyzed. Mean values are plotted; error bars represent SD.
NK cells isolated from a GS2 patient are impaired in the ability to recruit Munc13-4 to polarized perforin granules in PMA/ionomycin-activated cells. Resting NK cells were isolated from a patient, a healthy heterozygous parent, and a healthy donor, then were incubated alone or stimulated with PMA and ionomycin for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling with anti-Rab27a polyclonal antibody and anti-perforin mAb. Confocal images of single, representative resting (A) and PMA/ionomycin-stimulated (B) Rab27a-deficient cells are shown, with scale bars representing 5 μm. (C) Colocalization of perforin with Munc13-4 was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ. Ten to 15 cells per condition were analyzed. Mean values are plotted; error bars represent SD.
Engagement of LFA-1 induces Rab27a, whereas engagement of CD16 induces Munc13-4 recruitment to lytic granules
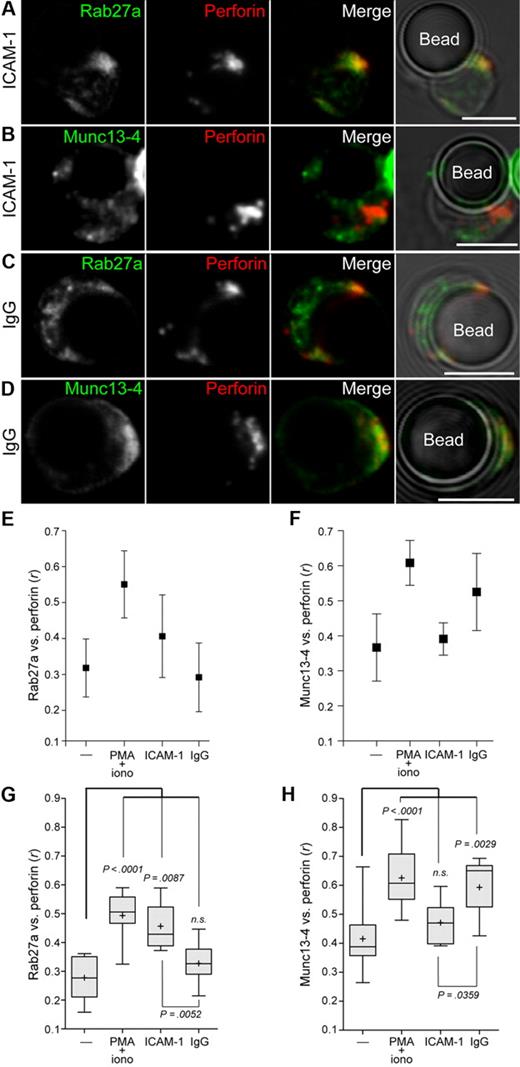
NK-cell cytotoxicity involves integration of signals from diverse receptors.23,42 Therefore, the next step was to investigate how individual NK-cell receptor engagement regulates the recruitment of Rab27a and Munc13-4 to lytic granules. Engagement of leukocyte functional antigen-1 (LFA-1; CD11a/CD18), a β2-integrin, controls lytic granule polarization but does not induce degranulation by resting NK cells.36 Conversely, engagement of CD16 induces lytic granule degranulation but not polarization.36 Resting NK cells were incubated with beads coated with recombinant human ICAM-1-Fc fusion protein or IgG for 20 minutes, then fixed, permeabilized, and labeled for perforin and either Rab27a or Munc13-4. Few conjugates formed between NK cells and beads that had not been coated with ligand. Unexpectedly, in conjugated NK cells, ICAM-1–coated beads induced colocalization of Rab27a, but not Munc13-4, with perforin (Figure 5A-B). In contrast, engagement of CD16 by IgG-coated beads did not induce colocalization of Rab27a with perforin (Figure 5C). However, engagement of CD16 induced Munc13-4 colocalization with perforin (Figure 5D). Colocalization between perforin and Rab27a or Munc13-4 was calculated as Pearson correlation coefficient, r, with one representative donor (Figure 5E-F) and cumulative data for 5 donors (Figure 5G-H). Relative to resting cells, LFA-1 engagement induced a significant increase in colocalization between Rab27a and perforin (0.28 ± 0.07 vs 0.46 ± 0.08; mean ± SD; Figure 5G), whereas CD16 engagement did not (0.28 ± 0.07 vs 0.33 ± 0.07). Conversely, CD16 engagement induced a significant increase in colocalization between Munc13-4 and perforin relative to resting cells (0.42 ± 0.11 vs 0.59 ± 0.10; Figure 5H), whereas LFA-1 engagement did not (0.42 ± 0.11 vs 0.47 ± 0.08). Thus, LFA-1 signals preferentially recruited Rab27a to perforin granules, whereas CD16 recruited Munc13-4.
Engagement of LFA-1 preferentially induces Rab27a colocalization with perforin in resting NK cells, whereas engagement of CD16 preferentially induces Munc13-4 colocalization with perforin in resting NK cells. Resting NK cells were incubated with recombinant human ICAM-1-Fc or human IgG-coated beads for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling. Cells conjugated with ICAM-1-Fc–coated beads were labeled with anti-perforin mAb and (A) anti-Rab27a polyclonal antibodies or (B) anti-Munc13-4 polyclonal antibodies. Cells conjugated with IgG-coated beads were labeled with anti-perforin mAb and (C) anti-Rab27a polyclonal Abs or (D) anti-Munc13-4 polyclonal Abs. Confocal images show single cells in conjugate with a bead and are representative of 5 independent experiments. Scale bars represent 5 μM. Colocalization between perforin and (E) Munc13-4 and (F) Rab27a antibody labeling in NK cells isolated from one representative healthy donor was measured and compared between resting NK cells, PMA and ionomycin-treated cells, and conjugates with IgG or ICAM-1–coated beads. Colocalization of the 2 fluorescent signals was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ. Ten to 15 cells per condition were analyzed, and mean r is graphed, with error bars representing SD. (G-H) Plots represent cumulative data from 10 to 15 cells from each of 5 donors. Plus symbols represent mean values, and error bars represent SD. Boxes represent the 25th, 50th, and 75th percentiles. Two-tailed paired Student t tests were performed to assess significance.
Engagement of LFA-1 preferentially induces Rab27a colocalization with perforin in resting NK cells, whereas engagement of CD16 preferentially induces Munc13-4 colocalization with perforin in resting NK cells. Resting NK cells were incubated with recombinant human ICAM-1-Fc or human IgG-coated beads for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling. Cells conjugated with ICAM-1-Fc–coated beads were labeled with anti-perforin mAb and (A) anti-Rab27a polyclonal antibodies or (B) anti-Munc13-4 polyclonal antibodies. Cells conjugated with IgG-coated beads were labeled with anti-perforin mAb and (C) anti-Rab27a polyclonal Abs or (D) anti-Munc13-4 polyclonal Abs. Confocal images show single cells in conjugate with a bead and are representative of 5 independent experiments. Scale bars represent 5 μM. Colocalization between perforin and (E) Munc13-4 and (F) Rab27a antibody labeling in NK cells isolated from one representative healthy donor was measured and compared between resting NK cells, PMA and ionomycin-treated cells, and conjugates with IgG or ICAM-1–coated beads. Colocalization of the 2 fluorescent signals was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ. Ten to 15 cells per condition were analyzed, and mean r is graphed, with error bars representing SD. (G-H) Plots represent cumulative data from 10 to 15 cells from each of 5 donors. Plus symbols represent mean values, and error bars represent SD. Boxes represent the 25th, 50th, and 75th percentiles. Two-tailed paired Student t tests were performed to assess significance.
Engagement of other NK-cell activation receptors differentially regulates Rab27a and Munc13-4 recruitment to granules
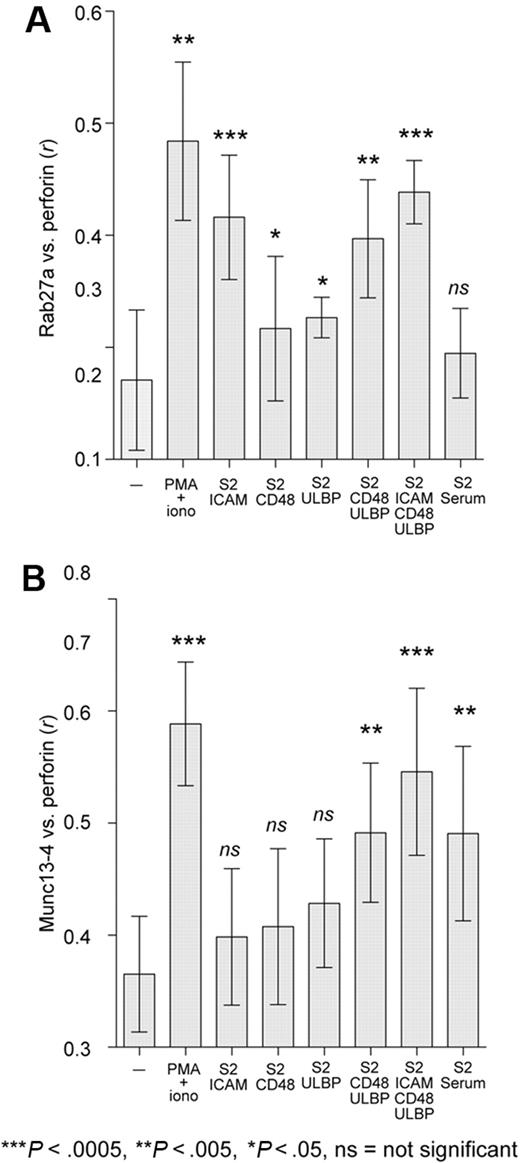
To examine a wider range of NK-cell receptors for natural cytotoxicity and compare results obtained using ligand-coated beads (Figure 5) with those from cells expressing freely diffusible ligands, we next used Drosophila S2 cells as targets for resting NK cells. S2 cells, which are not expected to express ligands for activating human NK-cell receptors, were transfected with one or more ligands for NK-cell–activating receptors, namely, ICAM-1, CD48, ULBP1, and combinations thereof.34 CD48 and ULBP1 bind receptors 2B4 and NKG2D on NK cells, respectively. To engage CD16 on NK cells, S2 cells were preincubated with human serum, which contains antibodies to oligosaccharide moieties expressed on S2 cells.43 Resting NK cells were mixed with S2 cells at a ratio of 1:1 and prepared for microscopy as for bead conjugates. Colocalization between perforin and Rab27a or Munc13-4 was calculated as Pearson correlation coefficient, r, for 10 to 15 cells, with data pooled from 3 donors (Figure 6). Recruitment of Rab27a and Munc13-4 in NK cells conjugated to S2-ICAM-1 or serum-precoated S2 cells corroborated results obtained with ICAM-1- and IgG-coated beads, respectively. Conjugation with S2-CD48 or S2-ULBP target cells caused a small but statistically significant increase in colocalization of Rab27a, but not Munc13-4, with perforin. S2-CD48-ULBP cells induced an increase in colocalization of both proteins with perforin. The largest increase in colocalization of both proteins was seen with S2 cells coexpressing ICAM-1, CD48, and ULBP1. Thus, individual engagement of the activating receptors LFA-1, 2B4, or NKG2D, signals that do not by themselves induce resting NK-cell degranulation,34 preferentially recruited Rab27a to perforin granules, whereas engagement of CD16 or coengagement of 2B4 and NKG2D, signals that are sufficient to induce resting NK-cell degranulation,34,36 favored recruitment of Munc13-4.
Engagement of activating receptors differentially recruits Rab27a and Munc13-4 to perforin granules in resting NK cells. Resting NK cells were incubated with S2 cells expressing human ICAM-1, CD48, or ULBP1 singly or in combination or coated with human serum, for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling. Rab27a (A) and Munc13-4 (B) colocalization with perforin was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ. Ten to 15 NK cells in conjugates were analyzed per condition, and data from 3 or 4 donors were compiled. Mean values are plotted; error bars represent SD. Two-tailed paired Student t tests were performed to assess significance.
Engagement of activating receptors differentially recruits Rab27a and Munc13-4 to perforin granules in resting NK cells. Resting NK cells were incubated with S2 cells expressing human ICAM-1, CD48, or ULBP1 singly or in combination or coated with human serum, for 20 minutes at 37°C, followed by fixation, permeabilization, and labeling. Rab27a (A) and Munc13-4 (B) colocalization with perforin was calculated as Pearson correlation coefficient, r, using the JACOP plugin in ImageJ. Ten to 15 NK cells in conjugates were analyzed per condition, and data from 3 or 4 donors were compiled. Mean values are plotted; error bars represent SD. Two-tailed paired Student t tests were performed to assess significance.
Discussion
Understanding the molecular pathophysiology of immune disorders such as HLH not only contributes to a better knowledge of the immune system but may also benefit diagnosis and treatment.44 In the present study, functional analysis and semiquantitative subcellular imaging of endogenous proteins in freshly isolated NK cells from GS2 patients, FHL3 patients, and healthy controls were used to gain insight into how Rab27a and Munc13-4 facilitate degranulation. The results demonstrate that NK-cell degranulation induced by both natural cytotoxicity and ADCC is dependent on Rab27a. Moreover, very little Rab27a or Munc13-4 colocalized with perforin in resting NK cells. Instead, NK-cell activation-induced myosin-dependent colocalization of these exocytic effector proteins with perforin, suggesting recruitment from heterotypic vesicular compartments. Remarkably, recruitment of Rab27a or Munc13-4 to perforin-containing granules was preferentially controlled by signals from different receptors, yet depended on the presence of the other. The results demonstrate that engagement of distinct receptors differentially regulates perforin granule maturation.
We found that degranulation induced by receptors for natural cytotoxicity or ADCC was defective in 3 Rab27a-deficient GS2 patients. In a GS2 patient carrying a homozygous RAB27A mutation, Gazit et al concluded that CD16-mediated ADCC was intact, whereas natural cytotoxicity against K562 cells or P815 cells in an NKp30-dependent manner (reverse ADCC with anti-NKp30 mAb) was impaired.27 Our results, however, suggest that Rab27a, like Munc13-4 and Stx11,19,30,31 are required for both natural cytotoxicity and ADCC. Remarkably, a few NK cells from Rab27a-deficient patients were able to degranulate at low levels. This residual degranulation may support killing of target cells in assays performed in vitro with high effector-to-target cell ratios and may account for the residual cytotoxicity in GS2 patients, as noted in this study and previous case reports.45,46 Thus, a less profound deficiency was observed in NK cells from GS2 patients, with residual degranulation and cytotoxicity present, in contrast with the complete loss of function in NK cells from FHL3 patients.30,31 This small residual degranulation could be the result of a degree of redundancy in Rab proteins for activation of Munc13-4. The somewhat less severe functional deficits in GS2 patients compared with FHL3 patients are consistent with later onset of disease and longer periods of disease-free remission in GS2 patients.31,45 These observations also concur with findings in mouse models of GS2 and FHL3.47,48 Thus, Rab27a may greatly increase the efficiency of granule fusion with the plasma membrane, but this fusion can still proceed at a low level in the absence of Rab27a.
In a seminal study of exocytosis in CTL, Menager et al described a lytic granule maturation process that requires cooperation of endosome-derived “exocytic vesicles” with lysosome-related, perforin-containing granules,32 contrary to previous findings suggesting constitutive association of Rab27a and Munc13-4 with secretory lysosomes and direct fusion of this compartment with the plasma membrane.29 In their model, anti-CD3 mAb activation of IL-2 cultured CTL induced Munc13-4–mediated fusion between Rab7+ Rab27a+ late endosomes and Rab11+ Munc13-4+ recycling endosomes, which then translocated along with lytic granules to the immune synapse, followed by a heterotypic fusion event between these 2 compartments and the plasma membrane adjacent to the site of granule exocytosis. This study was performed by overexpression of fluorescently tagged Rab27a and Munc13-4, along with Rab7 and Rab11. Overexpression of multiple membrane fusion-mediating proteins and chronic cytokine activation may alter cellular morphology and function, confounding analysis. Therefore, our findings demonstrating that engagement of activating receptors by physiologically ligands expressed on target cells can induce colocalization of endogenous Rab27a and Munc13-4 with perforin in freshly isolated, resting NK cells provide necessary support for some of the conclusions of Menager et al.32 Moreover, the punctate distribution of Rab27a and Munc13-4 in unstimulated NK cells and the observation that recruitment of these proteins to perforin granules is blocked by pharmacologic inhibition of MLCK indicate localization of the majority of Rab27 and Munc13-4 to heterologous vesicle compartments distinct from perforin granules. Our data reveal that myosin facilitates lytic granule maturation preceding plasma membrane fusion, extending findings that allege a requirement for myosin IIA in NK-cell degranulation.40 Together, these observations support the concept of lytic granule maturation through heterotypic vesicle fusion.32 Regrettably, labeling for organelle markers in resting NK cells was, in most cases, too weak to facilitate further characterization of the Rab27a+ and Munc13-4+ compartments (S.M.W. and Y.T.B., unpublished observations, February 2009).
Our results indicate that colocalization of Rab27a and Munc13-4 with perforin does not necessarily occur at sites adjacent to the target cell interface, in contrast with conclusions reached by Menager et al with transfected and cytokine-cultured cells.32 Stimulation with PMA and ionomycin induced colocalization of Rab27a and Munc13-4 with perforin at the microtubule organizing center centrally located within the cells. Importantly, recruitment of Rab27a and Munc13-4 to perforin was differentially regulated by signals from diverse receptors. Specific engagement of LFA-1 and, to a lesser extent, NKG2D or 2B4 by ligands expressed on target cells, promoted colocalization between perforin and Rab27a, but not Munc13-4. Conversely, engagement of CD16 preferentially recruited Munc13-4 to perforin granules, whereas coengagement of NKG2D and 2B4 synergy induced colocalization of both Rab27a and Munc13-4 with perforin. Together, the data demonstrate that recruitment of Rab27a and Munc13-4 to perforin granules does not necessarily follow a specific sequence but is guided by qualitatively different signals from distinct receptors. In these experiments, engagement of receptors that do not induce strong intracellular Ca2+ mobilization or degranulation preferentially recruited Rab27a to granules. In contrast, engagement of CD16 or NKG2D and 2B4 synergy, which readily induce intracellular Ca2+ mobilization and degranulation,42 induced colocalization of Munc13-4 with perforin.
How Rab27a and Munc13-4 facilitate secretory lysosome exocytosis remains unclear. Evidence for direct interactions in other hematopoietic cell types suggests that Munc13-4 is an effector of GTP-bound Rab27a and that they function in the same fusion step during granule exocytosis, after polarization of granules to the immune synapse.9,26,28,29 However, in resting NK cells, Rab27a localization to granules on activation is Munc13-4–dependent, as Rab27a is not recruited to polarized perforin granules on activation of NK cell from Munc13-4–deficient FHL3 patients. This inverse relationship between Rab27a and the effector Munc13-4 has also been reported for CTL.32 Thus, either Rab27a recruitment to, or retention at, the lytic granules is Munc13-4–dependent. Unexpectedly, induced colocalization of Munc13-4 with perforin was abrogated in NK cells from a GS2 patient, although polarization of perforin was not impaired. In resting cells from this patient, Munc13-4 displayed its normal punctate distribution, as predicted by the C-terminal Munc homology domain-containing portion of Munc13-4, not the N-terminal Rab27a binding domain, being the main determinant of membrane association.29 Therefore, reciprocally, either Munc13-4 recruitment to or retention at the lytic granules is Rab27a-dependent. Engagement of CD16 is sufficient for degranulation, albeit causing inefficient cytotoxicity.36 Our results demonstrate that CD16 signaling increases Munc13-4, but not Rab27a, colocalization with perforin. Thus, although CD16 preferentially induces Munc13-4 recruitment to perforin granules, low levels of Rab27a constitutively associated with perforin granules might facilitate CD16-induced degranulation. What then is the role of preferential Rab27a recruitment by receptors, such as LFA-1? A recent study has provided mechanistic insight to how engagement of LFA-1 facilitates efficient NK-cell cytotoxicity in conjunction with receptors that trigger degranulation.49 Engagement of LFA-1 is required for formation of an organized synapse with bidirectional vesicular traffic. Conceivably, in addition to the pivotal role of Rab27a in activation of Munc13-4 for degranulation, signals from LFA-1 and other receptors for accumulation of Rab27a on perforin granules might promote the architecture of such centrally organized trafficking structures through Munc13-4–independent mechanisms. In pancreatic β-cells, Rab27a has been implicated in facilitating refilling of releasable insulin granule pools.50 Recruitment of Rab27a may represent an early stage of immune synapse priming, with structural organization necessary for protein and membrane recycling, as well as granule fusion and release. In most physiologic conditions, ligands for receptors that recruit Rab27a are more widely expressed than those that induce strong intracellular Ca2+ mobilization, Munc13-4 recruitment, and degranulation. Thus, signals for Rab27a recruitment might precede those for Munc13-4 recruitment. The function of Munc13-4, recruited to perforin granules on engagement of receptors capable of inducing robust Ca2+ mobilization and granule release, could be more limited to regulation of the final plasma membrane fusion event.
Further mapping of the signaling pathways downstream of lymphocyte receptors promises to elucidate the complex regulation of lytic granule release by receptor signaling and cytoskeletal interactions. The assays presented herein for assessing lytic granule maturation in freshly isolated NK cells from patients may facilitate studies of patients with mutations in other genes that impair lytic granule release and cause hemophagocytic syndromes. Together, such studies should provide further insights into how Rab27a and Munc13-4 cooperate for cytotoxic lymphocyte exocytosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank E. Long for the gift of recombinant human ICAM-1-Fc.
S.M.W. was supported by an Australian Postgraduate Award and a Swedish Institute Guest Scholarship. This research was supported by the Swedish Research Council, Swedish Cancer Society, and Swedish Children's Cancer Foundation (H.-G.L., J.-I.H.), Swedish Foundation for Strategic Research (H.-G.L.), the Cancer and Allergy Foundation of Sweden, Stockholm County Council, Märta and Gunnar V Philipson Foundation (J.-I.H.), Mary Beve's Foundation, David & Astrid Hagelen's Foundation, Karolinska Institutet's Research Foundation (Y.T.B.), and Coordination Theme 1 (Health) of the European Community's FP7 (Grant Agreement no. HEALTH-F2-2008-201461).
Authorship
Contribution: S.M.W. designed and performed most of the experimental work, analyzed and interpreted data, and wrote the manuscript; M.M. designed and performed genetic analysis under the supervision of M.N.; S.C.C.C. performed experimental work; A.G.B., J.J.B., C.H., S.R., O.R., J.W., and J.-I.H. recruited study subjects; H.H. and J.L.S. contributed valuable reagents and provided input on the manuscript; J.-I.H. and H.-G.L. interpreted data and contributed to the manuscript; and Y.T.B. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephanie M. Wood, Center for Infectious Medicine, Department of Medicine, Karolinska Institutet, Karolinska University Hospital Huddinge, S-14186 Stockholm, Sweden; e-mail: Stephanie.Wood@ki.se; or Yenan T. Bryceson, Center for Infectious Medicine, Department of Medicine, Karolinska Institutet, Karolinska University Hospital Huddinge, S-14186 Stockholm, Sweden; e-mail: Yenan.Bryceson@ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal