Abstract
Tissue microenvironment and stroma-derived extracellular matrix (ECM) molecules play important roles in the survival and differentiation of cells. Mouse natural killer (NK) cells usually die within 24 hours once isolated ex vivo. Exogenous cytokines such as interleukin-12 (IL-12) and IL-15 are required to maintain the survival and activity of mouse NK cells cultured in vitro. Whether and how ECM molecules such as fibronectin can support the survival of NK cells remain unknown. We demonstrate that fibronectin, just like IL-15, can maintain survival of mouse NK cells in vitro. Furthermore, we show that fibronectin binds to the CD11b on NK cells, and then CD11b recruits and activates Src. Src can directly interact with β-catenin and trigger nuclear translocation of β-catenin. The activation of β-catenin promotes extracellular signal-related kinase (ERK) phosphorylation, resulting in the increased expression of antiapoptotic protein B-cell leukemia 2 (Bcl-2), which may contribute to the maintenance of NK-cell survival. Consistently, fibronectin cannot maintain the survival of CD11b− NK cells and β-catenin–deficient NK cells in vitro, and the number of NK cells is dramatically decreased in the β-catenin–deficient mice. Therefore, fibronectin can maintain survival of mouse NK cells by activating ERK and up-regulating Bcl-2 expression via CD11b/Src/β-catenin pathway.
Introduction
Natural killer (NK) cells are the most important effector cells of innate immunity against viral infection and tumors, and also play important roles in the initiation of adaptive immunity.1,2 In accordance with their powerful functions in immune surveillance and regulation of immune response, NK cells are distributed throughout lymphoid and nonlymphoid tissues.3,4 But, NK cells, especially mouse NK cells, usually die within 24 hours once separated ex vivo. Freshly isolated mouse NK cells, which are a type of short-lived lymphocyte in vitro, are difficult to extensively investigate in vitro unless their growth factors, such as interleukin-12 (IL-12) and IL-15, are added simultaneously to the culture system.5,6 However, IL-12 and IL-15 may affect the activity of NK cells, for example, IL-12 and IL-15 can enhance their cytotoxicity with increased expression of interferon-γ (IFN-γ) and perforin.5,7 So, identifying mechanisms that mediate the survival of NK cells and looking for the factors maintaining NK-cell survival without effect on their activity might be helpful to better understand the immunobiologic characteristics of NK cells in vivo. To a large extent, cell survival is controlled by the balance between proapoptotic and antiapoptotic members. It has been reported that Bim, Noxa, and myeloid cell leukemia 1 are key regulators of IL-15–dependent survival of mouse NK cells,8 and B-cell leukemia xL (Bcl-xL) is associated with the antiapoptotic effect of IL-15 on the survival of CD56(dim) NK cells.9 But the molecular details of NK-cell apoptosis and survival have not been well defined.
Tissue microenvironment and stroma-derived extracellular matrix (ECM) molecules have been shown to play important roles in the survival and differentiation of cells. Especially the interaction between ECM and integrin has been extensively studied for the survival, adhesion, and migration of stem cells and cancer cells.10-12 Fibronectin, a main component of ECM, is secreted by fibroblasts, chondrocytes, endothelial cells, macrophages, as well as certain epithelial cells.13 As an insoluble glycoprotein dimer, fibronectin serves as a linker in the ECM and is involved in many cellular processes, including cell adhesion/migration, tumor invasion and metastasis, tissue repair, and so on.13 Analysis of NK-cell adhesion to various extracellular matrix components demonstrates significant NK-cell adhesion to fibronectin but much less to laminin or collagens I and IV.14 It has also been reported that fibronectin increases NK-cell migration, and immobilized fibronectin enhances lymphokine-activated killer cell activity.15 One of our previous studies showed that liver stroma could chemoattract and adhere Toll-like receptor 3–triggered NK cells, and further augment the cytotoxicity and IFN-γ production of Toll-like receptor 3–triggered NK cells via fibronectin.16 In addition, we once demonstrated that splenic stroma-derived fibronectin, working together with stroma-derived transforming growth factor-β, can induce proliferation and further differentiation of mature dendritic cells (DCs) into regulatory DCs.17 These observations indicate that fibronectin may interact with NK cells intimately in vivo. However, whether and how fibronectin can maintain survival of NK cells has remained unclear until now.
In this study, we demonstrate that fibronectin, just like IL-15, can maintain survival of mouse NK cells in vitro. The mechanistic study shows that fibronectin maintains NK-cell survival by activating extracellular signal-related kinase (ERK) and up-regulating Bcl-2 expression via CD11b/Src/β-catenin pathway. Our results provide a new method to prepare and maintain NK-cell survival in vitro, which will be helpful to the functional study of mouse NK cells in vitro, and will contribute to the better understanding of the immunobiologic characteristics of mouse NK cells existing in the tissue microenvironment.
Methods
Mice and cell line
Female C57BL/6J(B6) mice were obtained from Joint Ventures Sipper BK Experimental Animal Co. The B6.Cg-Tg(Mx1-cre)1Cgn/J and B6.129-Ctnnb1tm2Kem/J transgenic mice were obtained from The Jackson Laboratory and intercrossed. The offspring inheriting both homozygous Mx1-Cre and the floxed allele (β-cateninfloxed/floxed) were administrated with polyinosinic-polycytidylic acid (poly(I:C), 250 μg/mouse) every other day for 5 times to obtain conditional β-catenin−/− mice as reported.18 Littermate mice that have either β-cateninfloxed/floxed or wild-type allele were used as controls. Animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of the Second Military Medical University. Human HEK293 cells were obtained from ATCC.
Reagents
Poly(I:C), Src inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), ERK inhibitors PD98059 and U0126, and PKH-26 were purchased from Sigma-Aldrich. Carboxyfluorescein succinimidyl ester (CFSE) was from Invitrogen. Recombinant mouse IL-15 was from R&D Systems. Mouse and human fibronectin and annexin V–fluorescein isothiocyanate apoptosis detection kit were from Calbiochem. Anti-DX5, anti-CD56, and anti-phycoerythrin (PE) magnetic microbeads were from Miltenyi Biotec. Fluorescein-conjugated antibodies specific for NK1.1, CD49b (DX5), CD3, and CD11b were from BD PharMingen. Antibodies specific to β-catenin, phospho (p)–β-catenin(Ser675), p-β-catenin(Tyr654), Src, and p-Src(Tyr418) were from Abcam. Anti–p-p38, anti–p-ERK, anti–p-c-Jun NH2-terminal kinase (JNK), anti–p-Tyr, anti–β-actin, and horseradish peroxidase–coupled secondary antibodies were from Cell Signaling Technology. jetPEI transfection reagent was from Polyplus Transfection. Genomic DNA Isolation Kits was purchased from Generay Biotechnology.
DNA preparation and analysis
To identify the β-catenin alleles and the Mx1-Cre transgene, DNA was isolated from tail biopsies of adult mice. Genomic DNA was distilled using Genomic DNA Isolation Kits and 0.5 μg genomic DNA was used for polymerase chain reaction. The 5′ and 3′ primers used for detecting the Cre gene were pCre1 (5′-ATG CCC AAG AAG AAG AGG AAG GT-3′) and pCre2as (5′-GAA ATC AGT GCG TTC GAA CGC TAG A-3′), which generate a 447-bp product. Sense primer RM41 (5′-AAG GTA GAG TGA TGA AAG TTG TT-3′) and antisense primer RM42 (5′-CAC CAT GTC CTC TGT CTA TTC-3′) were used to detect the β-catenin floxed allele, generating 324-bp and 221-bp products from the floxed and wild-type alleles, respectively. Mice that have both β-catenin floxed allele and cre transgene and littermate mice with wild-type genotype or only β-catenin floxed allele were injected with polyI:C (250 μg/mouse, intraperitoneally) every other day 5 times. Sixteen days after poly(I:C) administration, the mice were killed and the splenocytes and bone marrow cells were isolated to obtain genomic DNA. Different combinations of floxed and/or floxdel β-catenin alleles and confirmation of deleted β-catenin gene were identified by polymerase chain reaction using primers RM41, RM42, and RM43 (5′-TAC ACT ATT GAA TCA CAG GGA CTT-3′), resulting in products of 221 bp for the wild-type allele, 324 bp for the floxed allele, and 500 bp for the floxdel allele.
Preparation and culture of NK cells
Murine NK cells were freshly isolated from spleen. Splenocytes were blocked with anti-Fcγ receptor and rat serum and then incubated with anti-CD3 PE and anti-PE microbeads. After isolation with negative selection magnetic column, the negatively selected cells were harvested and labeled with anti-DX5 microbeads. CD3−DX5+ NK cells were isolated via positive selection through magnetic column. The purity of the isolated murine NK cells was more than 95%, as analyzed by fluorescence-activated cell sorting (FACS).
To purify the subsets of NK cells with different level of CD11b expression, splenocytes were labeled with anti-CD3, anti-DX5, anti-NK1.1, and anti-CD11b. Then the CD3−DX5+NK1.1+ CD11b++, CD3−DX5+NK1.1+ CD11b+, and CD3−DX5+NK1.1+ CD11b− cell subsets were sorted by MoFlo (Dako Cytomation) as described previously.19 The purity of the sorted cells was routinely more than 96%. All of the NK cells were cultured in endotoxin-free RMPI 1640 medium (PAA Laboratories) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in the presence or absence of fibronectin.
Cell labeling and adoptive transfer
NK cells sorted from β-cateninfloxed/floxed mice or β-catenin−/− mice were washed in phosphate-buffered saline (PBS) and labeled with CFSE or PKH-26, respectively. For CFSE labeling, cells at 5 × 106/mL were incubated in 2.5 μM CFSE in PBS for 10 minutes at 37°C. PKH-26 labeling (2 × 10−6 M dye) was performed according to the manufacturer's instructions. CFSE or PKH-26 labeling reaction was stopped by adding serum. Cells were subsequently washed 3 times using RPMI medium followed by 1 PBS wash. CFSE-labeled NK cells (2 × 106) and PKH-26 labeled NK cells (2 × 106) were mixed together and then adoptively transferred into wild-type mice. Blood leukocytes from recipient mice were collected and periphery NK-cell numbers were counted using FACS.
Flow cytometry
For analysis of cell-surface markers, flow cytometry was conducted on FACS LSRII and the data were analyzed with CellQuest or FACSDiva software (all from BD Biosciences), as described previously.20
Plasmid constructs and transfection
Plasmids encoding wild-type β-catenin (pcDNA3.0-β-catenin W), GFP tagged β-catenin (pcDNA3.0-β-catenin GFP), wild-type Src (pcDNA3.0-Src W), and Flag-tagged Src (pcDNA3.0-Src Flag) were constructed as described previously.21 All constructs were confirmed by DNA sequencing. For transfection, HEK293 cells were seeded into 6-well plates and transfected with jetPEI according to the manufacturer's instructions. The cells were used 40 hours later for further experiments.
Confocal microscopy
HEK293 cells were transiently cotransfected with β-catenin construct and Src construct, and plated on a glass coverslip in a 6-well plate. Sorted NK cells were cultured in the presence of fibronectin and collected at the indicated times. All samples were fixed and labeled with anti-Src and anti–β-catenin antibody, and observed with Leica laser scanning microscope equipment as described previously.22 The c-Fret technology was executed according to the Leica laser scanning software (TCS-SP2, Leica System).
Western blotting
NK cells cultured in the presence or absence of fibronectin were collected and lysed at the indicated times. Equal amounts of protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes. P-β-catenin, Src, p-Src, p-ERK, p-JNK, p-p38, and Bcl-2 were detected as described previously.24
Immunoprecipitation
HEK293 cells were transiently transfected with different types of β-catenin constructs and/or Src construct. Sorted NK cells were cultured in the presence of fibronectin and collected at the indicated times. Coimmunoprecipitated proteins were detected by Western blotting as described previously.21
Statistical analysis
Statistical significance was determined by Student t test, with a P value of .05 or less considered statistically significant.
Results
Fibronectin maintains the survival of mouse NK cells in vitro
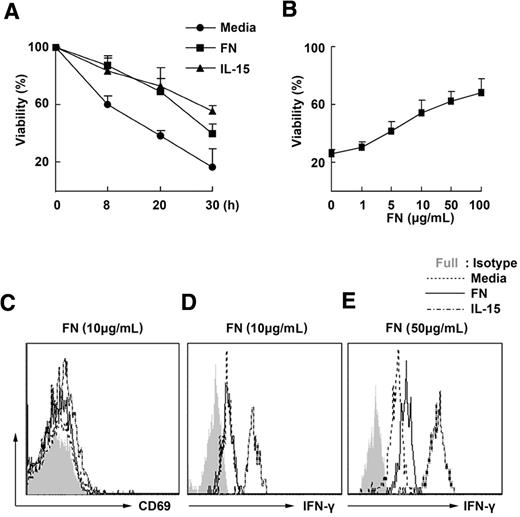
Stromal cells provide a supportive microenvironment for the survival of NK cells, whereas fibronectin is one of main products secreted by stromal cells. To investigate whether fibronectin is involved in the maintenance of the survival of NK cells, we cultured the freshly isolated mouse NK cells in the presence of mouse fibronectin and then observed the survival of NK cells. Without fibronectin, mouse NK cells died within 24 hours. Furthermore, it is apparent that fibronectin enhances the viability of mouse NK cells in vitro in a dose-dependent manner, just like the effect of IL-15, which is a well-known factor that elongates the life time of NK cells (Figure 1A-B). Interestingly, fibronectin at a concentration of 10 μg/mL did not affect the expression of CD69 and secretion of IFN-γ of mouse NK cells (Figure 1C-D), although 50 μg/mL fibronectin can slightly promote the secretion of IFN-γ by mouse NK cells (Figure 1E). We also observed that human fibronectin could maintain survival of human NK cells ex vivo (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results indicate that an appropriate concentration of mouse fibronectin, such as 10 μg/mL, maintains mouse NK-cell survival rather than affects the activity of mouse NK cells.
Fibronectin maintains the survival of mouse NK cells in vitro. (A) DX5+ natural killer (NK)–cell populations isolated from splenocytes of wild-type mice by magnetic-activated cell sorting were cultured in the presence or absence of fibronectin (FN, 10 μg/mL) or IL-15 (10 ng/mL), then labeled with annexin V and 7-amino-actinomycin D and assessed for their viability by FACS at the indicated times. (B) NK cells were cultured in the presence of fibronectin at different concentrations and their viability was detected using FACS 24 hours later. (C-D) NK cells were cultured for 24 hours in the presence of 10 μg/mL fibronectin and then CD69 expression on the NK cells was tested using FACS (C) and the level of IFN-γ in the supernatant was detected by cytometric bead array technology (D). (E) NK cells were cultured in the presence of 50 μg/mL fibronectin and then IFN-γ was detected 24 hours later. All data are shown as the mean ± SD of 3 independent experiments.
Fibronectin maintains the survival of mouse NK cells in vitro. (A) DX5+ natural killer (NK)–cell populations isolated from splenocytes of wild-type mice by magnetic-activated cell sorting were cultured in the presence or absence of fibronectin (FN, 10 μg/mL) or IL-15 (10 ng/mL), then labeled with annexin V and 7-amino-actinomycin D and assessed for their viability by FACS at the indicated times. (B) NK cells were cultured in the presence of fibronectin at different concentrations and their viability was detected using FACS 24 hours later. (C-D) NK cells were cultured for 24 hours in the presence of 10 μg/mL fibronectin and then CD69 expression on the NK cells was tested using FACS (C) and the level of IFN-γ in the supernatant was detected by cytometric bead array technology (D). (E) NK cells were cultured in the presence of 50 μg/mL fibronectin and then IFN-γ was detected 24 hours later. All data are shown as the mean ± SD of 3 independent experiments.
CD11b expression is required for the maintenance of NK cell survival by fibronectin
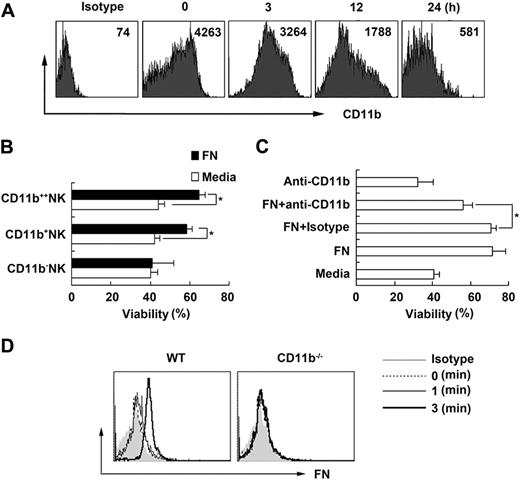
It is well known that ECM-integrin interactions are important for the survival and differentiation of many kinds of cells, and fibronectin, one of the ECM components, has several integrin-binding sites.24 Considering that CD11b is one of the receptors for fibronectin and also that CD11b is abundantly expressed on NK cells, we further investigated the role of CD11b in maintaining NK-cell survival by fibronectin. We found that the expression of CD11b on NK cells declined rapidly after NK cells separated ex vivo (Figure 2A), which was accompanied by NK-cell apoptosis. According to the expression intensity of CD11b, we sorted splenic NK1.1+DX5+ NK cells into 3 subpopulations of CD11b++, CD11b+, and CD11b− NK cells, then cultured them in the presence of fibronectin (supplemental Figure 2). We found that fibronectin could not maintain the survival of CD11b− NK cells but could maintain the survival of CD11b+ NK cells, with most pronounced effect on the survival of CD11b++ NK cells (Figure 2B). We further subdivided CD11b+ NK cells into several subsets and found that fibronectin could maintain the survival of CD11b+CD27− NK cells, CD11b+CD27+ NK cells, CD117+CD11b+ NK cells, and CD117−CD11b+ NK cells as well as CD11b+B220− NK cells, but not the survival of CD11b+B220+ cells (interferon-producing killer DCs; supplemental Figure 3). We also found that maintenance of NK-cell survival by fibronectin could be partially reduced when CD11b was blocked by neutralizing antibody (Figure 2C). To further confirm that fibronectin can bind with CD11b on NK cells, littermate control or CD11b−/− NK cells were cultured in the presence of fibronectin and then collected and washed and labeled with antifibronectin antibody at the indicated times. We found that there was fibronectin bound to NK cells derived from littermate control mice at 1 minute that soon disappeared at 3 minutes, but we did not detect binding of fibronectin to CD11b−/− NK cells (Figure 2D). These data further indicate that fibronectin could maintain the survival of NK cells though binding with CD11b on NK cells.
Fibronectin maintains NK-cell survival via CD11b. (A) The expression of CD11b on NK cells isolated from mouse splenocytes and cultured in vitro at the indicated times is shown. (B) Three subsets of splenic NK cells were sorted according to the expression of CD11b using MoFlo sorting system and cultured in the presence of fibronectin, and then viability was detected 24 hours later. (C) Sorted mouse NK cells were precultured with or without the neutralizing anti-CD11b antibody, then cultured in the presence of fibronectin and the viability was detected 24 hours later. Isotype indicates isotype control for antibody to CD11b. (D) NK cells sorted from CD11b−/− mice or littermate control were cultured in the presence of fibronectin and then collected, fixed, washed immediately at the indicated times, and labeled with antifibronectin antibody. The fibronectin bound to the surface of NK cells was tested by FACS. Data are shown as 1 typical result from 3 independent experiments with similar results or as mean ± SD of 3 independent experiments. *P < .05.
Fibronectin maintains NK-cell survival via CD11b. (A) The expression of CD11b on NK cells isolated from mouse splenocytes and cultured in vitro at the indicated times is shown. (B) Three subsets of splenic NK cells were sorted according to the expression of CD11b using MoFlo sorting system and cultured in the presence of fibronectin, and then viability was detected 24 hours later. (C) Sorted mouse NK cells were precultured with or without the neutralizing anti-CD11b antibody, then cultured in the presence of fibronectin and the viability was detected 24 hours later. Isotype indicates isotype control for antibody to CD11b. (D) NK cells sorted from CD11b−/− mice or littermate control were cultured in the presence of fibronectin and then collected, fixed, washed immediately at the indicated times, and labeled with antifibronectin antibody. The fibronectin bound to the surface of NK cells was tested by FACS. Data are shown as 1 typical result from 3 independent experiments with similar results or as mean ± SD of 3 independent experiments. *P < .05.
Fibronectin maintains the survival of NK cells via β-catenin pathway
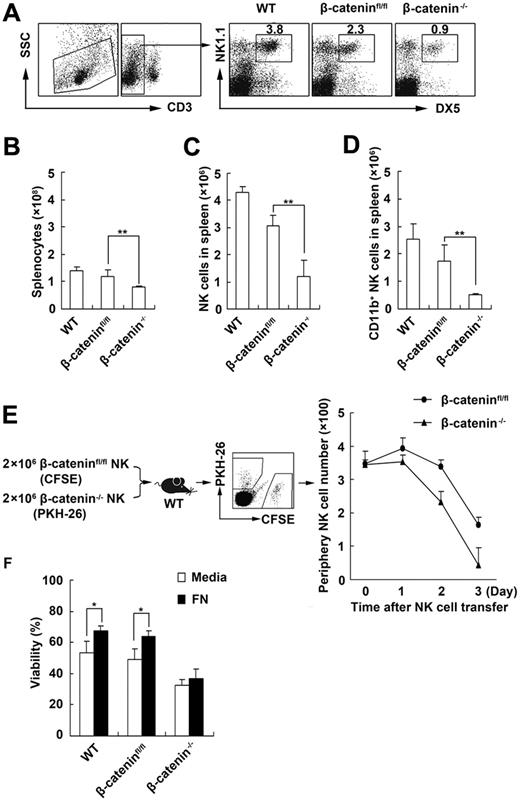
β-Catenin is a multifunctional molecule involving in cadherin-mediated cell-cell adhesion and Wnt signal pathway.25 It has been described that β-catenin not only plays a critical role in cell development and differentiation but also participates in maintaining immune cell survival.26 To investigate whether β-catenin is also involved in the maintenance of NK-cell survival, we first compared the proportions of NK cells in littermate control mice with β-catenin−/− mice and in vitro survival capability of the NK cells derived from littermate control mice and β-catenin−/− mice (supplemental Figure 4A). We found that the number and percentage of both splenic CD3−DX5+NK1.1+NK cells and CD11b+NK cells decreased in β-catenin−/− mice compared with those in littermate control mice (Figure 3A-D). Furthermore, we transferred the same number of CFSE-labeled β-cateninfloxed/floxed NK cells and PKH-26–labeled β-catenin−/− NK cells into wild-type mice and then detected peripheral CFSE+ and PKH-26+ NK cells in vivo at the indicated times. We found that the number of β-catenin−/− NK cells declined faster than that of β-cateninfloxed/floxed NK cells in vivo, indicating that viability of β-catenin−/− NK cells was low in vivo (Figure 3E). Moreover, we cultured the freshly isolated DX5+NK1.1+ NK cells of different origin with fibronectin in vitro and found that fibronectin maintained the survival only of NK cells from littermate control mice but not of NK cells from β-catenin−/− mice (Figure 3F, supplemental Figure 4B), whereas IL-15 could maintain the survival of β-catenin−/− NK cells (supplemental Figure 4C). These results indicate that β-catenin is critical for the maintenance of NK-cell survival, and fibronectin maintains the survival of NK cells via β-catenin pathway.
Fibronectin/CD11b maintains NK-cell survival via β-catenin. (A-C) The percentage (A) and number (C) of NK cells and CD11b+ NK cells (D) in spleen and the total number of splenocytes (B) of littermate control or β-catenin−/− mice were analyzed by FACS. WT and β-cateninfloxed/floxed (β-cateninfl/fl) mice were used as control mice. (E) The same number (2 × 106) of CFSE-labeled NK cells sorted from β-cateninfloxed/floxed mice and PKH-26–labeled NK cells sorted from β-catenin−/− mice were transferred to the wild-type mice. The CFSE+ or PKH-26+ NK1.1+ NK cells in 150 μL peripheral blood of the recipient mice were analyzed at the indicated times and counted using FACS. (F) The viability of NK cells derived from littermate control or β-catenin−/− mice after culture in the presence of fibronectin was tested with FACS. Data are shown as 1 typical result from 3 independent experiments with similar results or as mean ± SD of 3 independent experiments. *P < .05; **P < .01.
Fibronectin/CD11b maintains NK-cell survival via β-catenin. (A-C) The percentage (A) and number (C) of NK cells and CD11b+ NK cells (D) in spleen and the total number of splenocytes (B) of littermate control or β-catenin−/− mice were analyzed by FACS. WT and β-cateninfloxed/floxed (β-cateninfl/fl) mice were used as control mice. (E) The same number (2 × 106) of CFSE-labeled NK cells sorted from β-cateninfloxed/floxed mice and PKH-26–labeled NK cells sorted from β-catenin−/− mice were transferred to the wild-type mice. The CFSE+ or PKH-26+ NK1.1+ NK cells in 150 μL peripheral blood of the recipient mice were analyzed at the indicated times and counted using FACS. (F) The viability of NK cells derived from littermate control or β-catenin−/− mice after culture in the presence of fibronectin was tested with FACS. Data are shown as 1 typical result from 3 independent experiments with similar results or as mean ± SD of 3 independent experiments. *P < .05; **P < .01.
Src is required for the maintenance of NK-cell survival by fibronectin/β-catenin
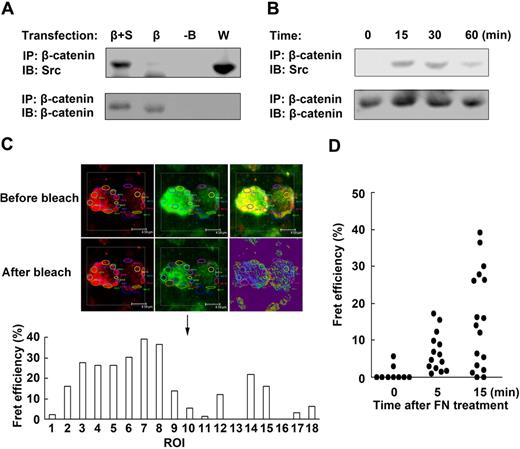
It has been reported that the activation of Src family is responsible for the phosphorylation of β-catenin of Tyr654 and regulation of E-cadherin/catenin association.27 So we wonder whether Src might act upstream of β-catenin and be responsible for the maintenance of NK-cell survival by fibronectin. To provide the direct evidence that Src induces the activation of β-catenin, we first analyzed whether Src could interact with β-catenin. HEK293 cells transfected with β-catenin construct and/or Src construct were lysed, and then equal amounts of proteins were immunoprecipitated with anti–β-catenin antibody, and immunoblot was performed to detect coprecipitated Src. As shown in Figure 4A, Src protein was coimmunoprecipitated with β-catenin. In addition, colocalization of Src and β-catenin was observed in the cytoplasm (supplemental Figure 5A). We further testified the interaction of Src and β-catenin by c-Fret technology using confocal imaging and found that, the donor emission within the region of interest (ROI) was augmented after acceptor photobleaching (supplemental Figure 5B), suggesting that Src directly interacts with β-catenin. Next we further tested whether fibronectin could induce Src/β-catenin complex formation in mouse NK cells. As shown in Figure 4B, almost no Src/β-catenin complex could be detected in the unstimulated NK cells. However, Src/β-catenin complex constitutively increased in NK cells within 30 minutes after fibronectin treatment. Furthermore, we also tested Src and β-catenin interaction induced by fibronectin in NK cells by c-Fret technology and observed that the Fret efficiency in the ROI was increased in NK cells after fibronectin treatment (Figure 4C-D). Thus, the results of the immunoprecipitation and Fret analysis suggest that fibronectin can induce Src and β-catenin interaction in mouse NK cells.
Src directly interacts with β-catenin. (A) Src was associated with β-catenin. HEK293 cells were transfected with PCDNA3.0 construct (-B), β-catenin construct (β), or β-catenin construct plus Src construct (β+S), respectively. The proteins were immunoprecipitated with anti–β-catenin antibody and blotted with anti-Src antibody. Protein in whole-cell lysate (W) was used as positive control. (B) Sorted NK cells cultured in the presence of fibronectin were collected at the indicated times. The proteins were immunoprecipitated with anti–β-catenin antibody and blotted with anti-Src antibody. (C) Example profiles of the fluorescence of donor (red) and acceptor (green) before and after photobleaching. The area circled by different color line was ROI. The Fret efficiency of the different ROI was shown in the histograph below the profiles. Objective 63×, numeric aperture 1.4. (D) The interaction of β-catenin and Src was testified by c-Fret technology in mouse NK cells after fibronectin treatment. The Fret efficiency of ROI of NK cells was dotted. Data are shown as 1 typical result from 3 independent experiments with similar results.
Src directly interacts with β-catenin. (A) Src was associated with β-catenin. HEK293 cells were transfected with PCDNA3.0 construct (-B), β-catenin construct (β), or β-catenin construct plus Src construct (β+S), respectively. The proteins were immunoprecipitated with anti–β-catenin antibody and blotted with anti-Src antibody. Protein in whole-cell lysate (W) was used as positive control. (B) Sorted NK cells cultured in the presence of fibronectin were collected at the indicated times. The proteins were immunoprecipitated with anti–β-catenin antibody and blotted with anti-Src antibody. (C) Example profiles of the fluorescence of donor (red) and acceptor (green) before and after photobleaching. The area circled by different color line was ROI. The Fret efficiency of the different ROI was shown in the histograph below the profiles. Objective 63×, numeric aperture 1.4. (D) The interaction of β-catenin and Src was testified by c-Fret technology in mouse NK cells after fibronectin treatment. The Fret efficiency of ROI of NK cells was dotted. Data are shown as 1 typical result from 3 independent experiments with similar results.
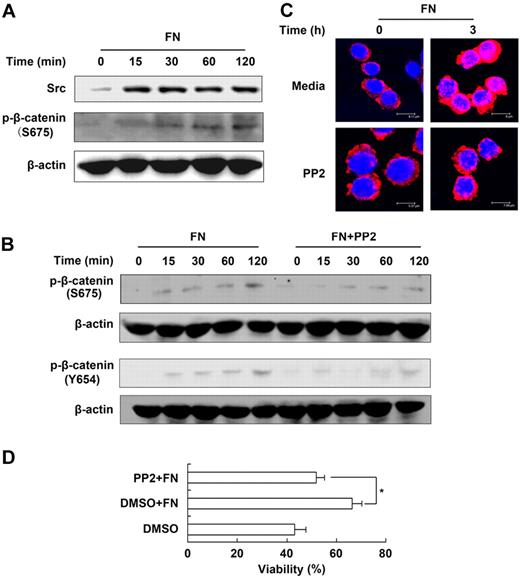
As shown in Figure 5A, fibronectin stimulation induced significant and rapid accumulation of constitutive Src, and induced the Ser675 and Tyr654 (Figure 5B) phosphorylation of β-catenin (the activated form of β-catenin28 ). Moreover, we pretreated NK cells with PP2, a selective Src inhibitor, and found that the phosphorylation of β-catenin of either Tyr654 or Ser675 was decreased and the level of nuclear β-catenin was reduced in the NK cells cultured in the presence of fibronectin (Figure 5B-C). Accordingly, the maintaining effect of fibronectin on NK-cell survival was blocked (Figure 5D, supplemental Figure 6A). The data demonstrated that Src, as an upstream molecule that activates β-catenin, participates in β-catenin–dependent NK-cell survival maintained by fibronectin.
Src is required for β-catenin–dependent NK-cell survival maintained by fibronectin. (A) The activation and expression of Src and β-catenin in NK cells cultured in the presence of fibronectin were analyzed by Western blot. (B) Expression of p-β-catenin of S675 and Y654 in NK cells cultured in the presence of fibronectin with or without pretreatment of PP2. (C) Nuclear translocation of β-catenin in NK cells cultured in the presence of fibronectin with or without pretreatment of PP2 was given by confocal imaging. Objective 63×, numeric aperture 1.4. (D) Viability of NK cells cultured in the presence of fibronectin with or without pretreatment of PP2 for 24 hours was tested using FACS. Data are shown as 1 typical result from 3 independent experiments with similar results or as mean ± SD of 3 independent experiments. *P < .05.
Src is required for β-catenin–dependent NK-cell survival maintained by fibronectin. (A) The activation and expression of Src and β-catenin in NK cells cultured in the presence of fibronectin were analyzed by Western blot. (B) Expression of p-β-catenin of S675 and Y654 in NK cells cultured in the presence of fibronectin with or without pretreatment of PP2. (C) Nuclear translocation of β-catenin in NK cells cultured in the presence of fibronectin with or without pretreatment of PP2 was given by confocal imaging. Objective 63×, numeric aperture 1.4. (D) Viability of NK cells cultured in the presence of fibronectin with or without pretreatment of PP2 for 24 hours was tested using FACS. Data are shown as 1 typical result from 3 independent experiments with similar results or as mean ± SD of 3 independent experiments. *P < .05.
β-Catenin–dependent ERK activation is required for the maintenance of NK-cell survival by fibronectin
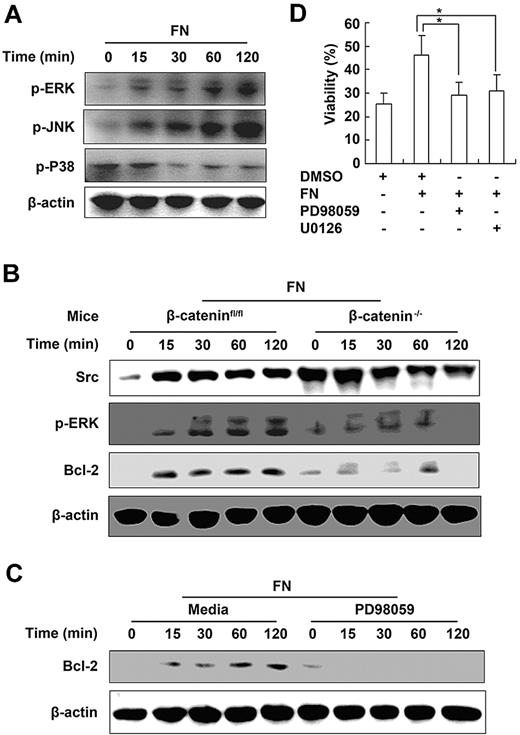
It has been reported that activation of ERK pathway and subsequent induction of antiapoptotic proteins such as Bcl-2 play important roles in the survival of CD8+ T cells.29 To further investigate the signaling mechanisms of β-catenin–dependent NK- cell survival mediated by fibronectin, we detected the activation of mitogen-activated protein kinases and expression of antiapoptotic protein Bcl-2 in NK cells cultured in the presence of fibronectin. We found that fibronectin induced activation of ERK and JNK and enhanced expression of Bcl-2 in NK cells (Figure 6A). In contrast, a lower level of phosphorylated ERK and decreased expression of Bcl-2 were observed in β-catenin−/− NK cells cultured in the presence of fibronectin (Figure 6B). Furthermore, we pretreated wild-type NK cells with ERK inhibitor PD98059 or U0126, respectively, then cultured them with fibronectin. We found that the expression of Bcl-2 in NK cells was decreased after ERK inhibitor treatment (Figure 6C), whereas the survival of NK cells maintained by fibronectin was blocked (Figure 6D, supplemental Figure 6B). Thus, these results demonstrate that β-catenin–dependent ERK activation is critical for the fibronectin-mediated NK-cell survival by up-regulating the expression of antiapoptotic protein Bcl-2.
β-Catenin–dependent activation of ERK is required for the maintenance of NK-cell survival by fibronectin. (A) Mitogen-activated protein kinases pathway in NK cells cultured in the presence of fibronectin was analyzed by Western blot. (B) Analysis of β-catenin, Src, p-ERK, and Bcl-2 in NK cells derived from littermate control (β-cateninfloxed/floxed) or β-catenin−/− mice and cultured in the presence of fibronectin. (C) Expression of Bcl-2 in NK cells cultured in the presence of fibronectin with or without pretreatment of PD98095 was tested by Western blot. (D) NK cells were pretreated with PD98095 or U0126 for 30 minutes, then cultured in the presence of fibronectin for the indicated times and viability of NK cells was analyzed using FACS. Data are shown as 1 typical result from 3 independent experiments with similar results or as mean ± SD of 3 independent experiments. *P < .05.
β-Catenin–dependent activation of ERK is required for the maintenance of NK-cell survival by fibronectin. (A) Mitogen-activated protein kinases pathway in NK cells cultured in the presence of fibronectin was analyzed by Western blot. (B) Analysis of β-catenin, Src, p-ERK, and Bcl-2 in NK cells derived from littermate control (β-cateninfloxed/floxed) or β-catenin−/− mice and cultured in the presence of fibronectin. (C) Expression of Bcl-2 in NK cells cultured in the presence of fibronectin with or without pretreatment of PD98095 was tested by Western blot. (D) NK cells were pretreated with PD98095 or U0126 for 30 minutes, then cultured in the presence of fibronectin for the indicated times and viability of NK cells was analyzed using FACS. Data are shown as 1 typical result from 3 independent experiments with similar results or as mean ± SD of 3 independent experiments. *P < .05.
Discussion
The extracellular matrix is a network of nonliving tissue that provides support to cells. The extracellular matrix also helps cells to bind together and regulates several cellular functions, such as adhesion, migration, proliferation, and differentiation.30 A series of our previous studies focused on the role of stromal microenvironment in the differentiation and functional regulation of immune cells. We demonstrate that cell-to-cell contact with stromal cells promoted mature DCs to proliferate in a fibronectin-dependent way, and that both stromal cell contact and stromal cell–derived transforming growth factor-β were required for their differentiation into a new regulatory DC subset.17,31 In addition, splenic stroma could induce differentiation of hematopoietic stem cells into regulatory DCs, and liver stroma and pulmonary stroma could induce differentiation of regulatory DCs.19,31,32 However, few reports are about the effect of stromal microenvironment and extracellular matrix molecules on the survival and function of NK cells to date. In this study, we identified fibronectin as the survival factor for mouse NK cells. Interestingly, fibronectin maintains NK- cell survival by activating ERK and up-regulating Bcl-2 expression via CD11b/Src/β-catenin pathway.
It is well known that β2 integrin CD11b/CD18 is an integral membrane protein that is present in the plasma membrane and secondary granules of neutrophils and serves as a receptor for a diverse group of ligands, including fibronectin, iC3b, intercellular adhesion molecule-1, β-glucan, coagulation factor X, and fibrinogen, and is critically involved in adhesion locomotion, chemotaxis, phagocytosis, and survival of cells such as eosinophils and neutrophils.33-35 More recently, Chiossone et al subdivided mouse NK cells into 4 subsets according to the surface density of CD27 and CD11b and proposed a 4-stage model of NK-cell maturation as follows: CD11blowCD27low→CD11blowCD27high→CD11bhighCD27high→CD11bhighCD27low NK cells.36 This model suggests that CD11b may be involved in the development and differentiation of NK cells. However, the effect of CD11b on NK survival has not been reported. Here, we subdivided NK cells into 3 subpopulations according to the expression level of CD11b, and found that fibronectin can maintain survival only of CD11b+ NK cells. To confirm that fibronectin can bind to CD11b of NK cells and then CD11b mediates the promotion of NK-cell survival by fibronectin, we sorted NK cells from CD11b−/− mice or littermate control mice and cultured them with fibronectin, then labeled them with antifibronectin antibody. We found that there was high level of fibronectin bound to NK cells derived from CD11b+/+ mice, in contrast, we did not detect the significant binding of fibronectin to CD11b−/− NK cells. We also went further to analyze the effect of neutralizing anti-CD11b antibody on the maintenance of NK- cell survival by fibronectin, and found that maintaining NK-cell survival by fibronectin could be partially reduced when CD11b was blocked by neutralizing antibody. These experiments further confirm that prosurvival effect on NK cells is mediated by FN binding to CD11b.
β-Catenin is a central component of the cadherin cell adhesion complex and plays an essential role in Wnt signaling cascade. In steady state, β-catenin associates with E-cadherin and the dissociative β-catenin is maintained at low levels through degradation by the proteosome. Upon receipt of a Wnt signal, Disheveled protein prevents degradation of β-catenin. Stabilized β-catenin enters the nucleus and combines with T-cell factor (TCF)/lymphoid enhancer factor (LEF) transcription factors, leading to the transcription of Wnt target genes such as c-myc, cyclin D, CD44, MMP7, IL-18, and others.37,38 This signal transduction pathway regulates many cellular and developmental processes, including cell proliferation, cell fate decision, and differentiation. As reported, the Wnt/β-catenin pathway plays an important role in immune cell development and survival. Differentiation of T cells and NK cells is greatly impaired in the absence of LEF1/TCF proteins, and also pro–B-cell proliferation is regulated by Wnt signaling.39 Furthermore, stabilization of β-catenin enhances double-positive thymocyte survival via up-regulating Bcl-xL.40 It has been also reported that NK cells expressing Ly-49A depend on the expression of a TCF-1 isoform, which includes a domain known to associate with the TCF-1 coactivator β-catenin.41 We adoptively transferred CFSE-labeled β-cateninfloxed/floxed NK cells and PKH-26–labeled β-catenin−/− NK cells to wild-type mice and then detected peripheral CFSE+ and PKH-26+ NK cells of the recipient mice at different time points after transferring. We found that β-catenin−/− NK cells died more easily in vivo than controls. In this study, we for the first time provide the mechanistic explanation for the role of β-catenin in NK-cell survival by showing that fibronection/CD11b recruits Src that may bind and activate β-catenin, and the activated β-catenin subsequently activates ERK pathway and in turn up-regulates Bcl-2 expression, finally resulting in the maintenance of NK-cell survival by fibronectin.
Members of Src family exhibit a conserved domain organization, which includes a myristoylated N-terminal segment, followed by SH3, SH2, linker and tyrosine kinase domains, and a short C-terminal tail.42 The Src family proteins regulate fundamental cellular process, including cell growth, differentiation, shape, migration, and survival and specialized cell signals. It has been reported that Src family is involved in CD11b-mediated signal pathway.42 In this study, we found that fibronectin promotes NK-cell survival via membrane receptor CD11b, and the intracellular protein Src is the key factor and the upstream molecule for β-catenin in NK-cell survival. Using immunoprecipitation and c-Fret technology, we found that Src interacts with β-catenin and thus induces the activation and nuclear translocation of β-catenin in mouse NK cells after fibronectin treatment. We showed that FN binding induced the formation of a Src/β-catenin complex in NK cells, and β-catenin phosphorylation at both Tyr654 and Ser675 decreased after treatment with Src inhibitor PP2. As a tyrosine kinase, Src can induce Tyr654 phosphorylation. However, until now, phosphorylation of only Ser675, but not Tyr654, has been shown to stabilize β-catenin and induce Tcf/Lef-dependent transcription. Therefore, how Ser675 phosphorylation is induced and the role of Tyr654 phosphorylation of β-catenin in the mediation of NK-cell survival need to be investigated in the future. Thus, we speculate that Src may phosphorylate β-catenin of Ser675 indirectly, ultimately leading to TCF/LEF-dependent transcription. Considering that β-catenin plays a role in cell-cell and cell-ECM adhesion, we speculate that Src/β-catenin pathway may be involved in signaling transduction among the interaction of ECM and NK cells that is important for the survival, differentiation, and function of NK cells.
The PI3K-Akt pathway and ERK signal pathway have been well studied and shown to regulate the Bcl-2 family of proteins in cell apoptosis. Therefore, the mechanism underlying NK-cell survival by fibronectin is due to activation of Src, the upstream signal molecule for β-catenin, and ultimately induces up-regulation of the expression of Bcl-2. What is the downstream signal pathway of β-catenin for Bcl-2 up-regulation? We found phosphorylation of ERK was down-regulated in β-catenin−/− NK cells after fibronectin stimulation. Using the ERK inhibitor, we confirmed that activation of ERK by β-catenin was responsible for Bcl-2 up-regulation in NK cells.
In conclusion, we here provide a possible mechanistic model by which fibronectin maintains survival of mouse NK cells as follows: stromal cells of lymph and nonlymph organ microenvironment secret fibronectin that binds CD11b on NK cells and then activates Src-β-catenin-ERK pathway, finally resulting in the up-regulation of Bcl-2, which contributes to the maintenance of NK-cell survival by fibronectin.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jingxia Jiang and Ming Yao for technical assistance; and Dr Huazhang An, Dr Taoyong Chen, and Dr Nan Li for their helpful discussions.
This work was supported by grants from the National Natural Science Foundation of China (30672386, 30572121, 30721091) and National Key Basic Research Program of China (2007CB512403, 2009CB522402).
Authorship
Contribution: T.Z. designed research, performed experiments, analyzed data, and wrote the paper; S.L. performed experiments, analyzed data, and wrote the paper; P.Y., C.H., J.W., J.L., Y.H., and Y.Y. performed experiments; and X.C. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xuetao Cao, Institute of Immunology, Zhejiang University School of Medicine, Hangzhou 310058, People's Republic of China; e-mail: caoxt@public3.sta.net.cn.
References
Author notes
T.Z. and S.L. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal