Blood group antigen immunogenicity rates are calculated by factoring in the ability to detect certain red cell antibodies lost over time. This new mathematical model could replace the formula established in 1961.
The likelihood of an antigen to induce antibodies in an exposed person is termed the antigen's immunogenicity. As medical decisions in transfusion and transplantation medicine could include the immunogenicity and affect the outcome as well as the cost of therapy, a precise estimate for immunogenicity is warranted.
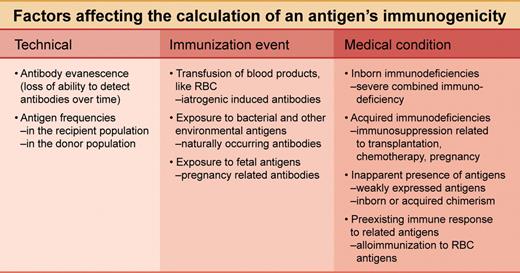
Factors affecting the calculation of an antigen's immunogenicity. Professional illustration by A. Y. Chen.
Factors affecting the calculation of an antigen's immunogenicity. Professional illustration by A. Y. Chen.
The immunogenicity is influenced by the immunization event, such as the route of exposure, and underlying medical conditions, such as immunosuppression (see figure). A pre-existing immune response to a red cell antigen could also correlate with an increased likelihood to mount an immune response to an additional red cell antigen.
It has been known since the 1950s that the prevalence of a red cell antibody poorly reflects the immunogenicity of the antigen. The antigen frequencies in the donor and recipient populations are a crucial technical factor to be considered in calculating immunogenicity. A formula devised by Giblett in 1961 corrected for these antigen frequencies and has since been applied to immunogenicity determinations.1
The Giblett formula, however, failed to correct for a second technical factor, the well-known loss of the ability to detect certain red cell antibodies over time. In this issue of Blood, Tormey and Stack have dubbed this loss of ability “antibody evanescence”2 and provide in their current study a formula that corrects for this evanescence.3 By applying their previously published retrospective data for relative antibody persistence,2 the authors establish new figures for the immunogenicity of common red cell antigens. It is likely that textbooks of serology and transfusion medicine will cite the study and quote its figures.
In a second novel feature of the current study, calculation of immunogenicity is derived only from observations of antibody occurrence in men and from antibodies not defined as naturally occurring. This approach, of course, restricts the calculated immunogenicity to iatrogenic immunization events (see figure). This restriction is important. These specific iatrogenic events could be avoided by exposure to antigen-matched blood products, if deemed necessary in transfusion therapy. The authors provide important data for reaching this decision. Although differentiation among these 3 different routes of challenge has previously been blurred, immunization resulting from exposure to fetal and environmental antigens is generally not a consequence of medical decisions.
Genotyping for blood groups will eventually facilitate the “dry matching” of many antigens inexpensively.4 When this technical advance becomes widely available, our understanding of immunogenicity should be developed enough to allow a conscious decision as to which antigens to match and in what order to most benefit patients with specific medical conditions.
The authors implemented the iatrogenic immunization event as a term in their new formula. One might have preferred to account only for antigen frequencies and evanescence in the formula, incorporating the topics of immunization events and the definition of naturally occurring antibodies into the design of the clinical study underlying the data used for calculation. The formula would have been handier.
Immunization events other than those associated with blood transfusion are important and deserve attention. Immunogenicity of an antigen may differ among the possible routes of challenge, allowing interesting insights into the biology of immune response. A calculation accounting for antigen frequencies and evanescence but applied specifically to immunization during pregnancy or by environmental antigens (for instance, in probiotics)5 would be interesting.
A huge number of patients are transfused on a routine basis. If the means were available and attention paid, clinical evidence for immunogenicity gained could be gathered and analyzed more comprehensively. Using the right tools, like the current formula, prospectively collected clinical data from carefully defined immunization events and well-documented medical conditions would likely yield new insights. Such data are difficult or impossible to generate in humans by any other clinical study approach because of cost and ethical concerns. The data would improve our understanding of human immunology and, at the same time, even benefit patient care at a relatively modest, if any, added burden to the health care system.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal