On page 2064 in the September 1, 2008, issue, there is an error in Figure 1A. Lanes indicating interactions between HES1 constructs and FA proteins are inverted. The correct Figure 1 is shown.
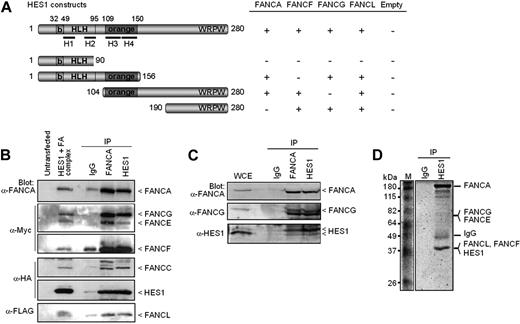
FA proteins interact with HES1. (A) Yeast-2-hybrid assay with HES1 and FA proteins. Yeast strain AH109 was cotransformed with HES1 constructs expressing full-length or truncated HES1 protein with FA proteins as indicated and assayed for interaction as described in “Yeast-2-hybrid.” A positive interaction is indicated as +. Negative controls included pGBK-HES1 cotransformed with pGADT7 empty vector. (B) FA core complex components coimmunoprecipitate with HES1. 293T cells were cotransfected with HA-tagged HES1 and FA coding vectors and were subjected to immunoprecipitation (IP) with either anti-FANCA antibodies, anti-HES1 antibodies, or control IgG. IP was analyzed by SDS-PAGE and Western blotting using the indicated antibodies. (C) Co-IP of endogenous proteins from 293T cell extracts using anti-HES1 or anti-FANCA antibodies. (D) Coomassie gel staining of endogenous protein extracts of 293T cells subjected to IP using anti-HES1 antibodies. Major bands were extracted from gel slices and the indicated proteins were identified by mass spectrometry.
FA proteins interact with HES1. (A) Yeast-2-hybrid assay with HES1 and FA proteins. Yeast strain AH109 was cotransformed with HES1 constructs expressing full-length or truncated HES1 protein with FA proteins as indicated and assayed for interaction as described in “Yeast-2-hybrid.” A positive interaction is indicated as +. Negative controls included pGBK-HES1 cotransformed with pGADT7 empty vector. (B) FA core complex components coimmunoprecipitate with HES1. 293T cells were cotransfected with HA-tagged HES1 and FA coding vectors and were subjected to immunoprecipitation (IP) with either anti-FANCA antibodies, anti-HES1 antibodies, or control IgG. IP was analyzed by SDS-PAGE and Western blotting using the indicated antibodies. (C) Co-IP of endogenous proteins from 293T cell extracts using anti-HES1 or anti-FANCA antibodies. (D) Coomassie gel staining of endogenous protein extracts of 293T cells subjected to IP using anti-HES1 antibodies. Major bands were extracted from gel slices and the indicated proteins were identified by mass spectrometry.
On page 2067, under the section “HES1 is required for MMC resistance and FANCD2 monoubiquitination” (which begins on page 2066), there are errors in the first sentence of the last paragraph that reads, “Among all FA complementation groups, only cells with mutations in the downstream components (FANCD1/BRCA2, FANCJ/BRIP1/BACH1, and FANCN/PALB2) have an impaired Rad51 foci phenotype.38,43” The correct sentence should read: “Among all FA complementation groups, only cells with mutations in the downstream components (FANCD1/BRCA2 and FANCN/PALB2) have an impaired Rad51 foci phenotype.37-39,43”

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal