Abstract
In humans, approximately 3% of peripheral CD8+ T cells coexpress CD4 dimly on their surface and hence are designated as CD4dimCD8bright T cells. We evaluated the contribution of this CD4dimCD8bright T-cell population to anti-HIV immunity. We demonstrate that CD4dimCD8bright T cells generate greater than 55% of CD8+ T-cell antigen recognition and effector response to HIV, as evaluated by multiple parameters for assessing T-cell antiviral immunity, including HIV tetramer recognition, cytokine production, and cytolytic potential. Inhibition of major histocompatibility class II (MHC-II) on target cells or CD4 on CD4dimCD8bright T cells diminishes their anti-HIV responses, suggesting that CD4 on effector cells and MHC-II on target cells provides an additional arm of contact between effector and target cells which is critical to CD4dimCD8bright T-cell function. CD4dimCD8bright T cells also exhibit features that are indicative of central memory T cells. Finally, CD4dimCD8bright T cells are elevated in blood of HIV+ long-term nonprogressors in comparison to HIV− donors. Collectively, our findings show that CD4dimCD8bright T cells designate an enriched antiviral subpopulation of CD8+ T cells that should be targeted for therapeutic intervention or evaluation of vaccine efficacy.
Introduction
CD8+ T cells mount protective immune responses against viruses by killing infected cells or secreting antiviral cytokines. In HIV infection, loss or dysregulation of CD8+ T cells is correlated with disease progression.1-3 Although expression of CD8 or CD4 on mature T cells is thought to be mutually exclusive, defining cytotoxic and helper cells, respectively, considerable evidence from our laboratory4-6 and others7-12 has shown that CD4 is coexpressed on a subset of CD8+ T cells. In the periphery, CD4-expressing CD8+ T cells constitute 3% to 5% of CD8+ T cells and 1% to 3% of lymphocytes.13,14 Within these cells is a CD4dimCD8bright T-cell subpopulation, whose CD4 expression is dim (lower than CD4 expression on CD4+ helper T cells) and the CD8 expression is bright (equal to or greater than CD8 expression on conventional CD8+ cytotoxic T cells).
Several lines of evidence have shown that CD4dimCD8bright T cells are not an artifact but represent a genuine, mature subset of CD8+ T cells. (1) CD4-depeleted CD8+ T cells after anti-CD3/CD28 or superantigen staphylococcus enterotoxin B (SEB) stimulation up-regulate CD4 on their surface, as evaluated by mRNA and protein expression.4,7,8 This level of CD4 induction on CD8+ T cells in vitro is substantial because 30% to 60% of purified CD8+ T cells re-express CD4 after anti-CD3/CD28 or SEB stimulation.4 (2) CD4dimCD8bright T cells are not prematurely released thymocytes, because they are negative for thymocyte marker CD1a.4 (3) The T-cell receptor (TCR) of CD4dimCD8bright T cells is αβ and their CD8 molecule is αβ,4 which confirms that CD4dimCD8bright T cells are not gut-derived CD8+ T cells, which are predominantly TCRγδ and CD8αα. (4) These T cells do not express CD16, CD56, or 6B11 and therefore are not natural killer T (NKT) cells.6
CD4dimCD8bright T cells are not unique to humans. They are prominent in animal species including swine, rodent, chicken, and monkey.15 Their frequency is also elevated in clinical conditions, including human T lymphotropic retrovirus type 1,16 chronic T lymphoid leukemia,17 Sjögren syndrome,18 myasthenia gravis,19 multiple sclerosis,20 idiopathic thrombocytopenic purpura,21 and Behçet syndrome.22
Although the exact role of CD4dimCD8bright T cells in these conditions is not clear, their up-regulation suggests a significant role in host immunity. We have previously demonstrated that CD4dimCD8bright T cells are more activated than CD4−CD8+ T cells.4 Others have shown that CD4 expression on CD8+ T cells enhances their IFNγ and Fas ligand expression,10 whereas, inversely, lack of CD4 expression on CD8+ T cells from CD4-knockout mice leads to decreased CD8+ T-cell responses against lymphocytic choriomeningitis virus (LCMV).11 The contribution of CD4dimCD8bright T cells to anti-HIV immunity, however, is not clear.
We evaluated here the role of CD4dimCD8bright T cells in anti-HIV immunity. We demonstrate that, although CD4dimCD8bright T cells represent a minor percentage of the total CD8+ T cells, they are enriched for anti-HIV–specific responses. This phenomenon is not restricted to HIV, because these cells are also enriched for cytomegalovirus (CMV)–specific responses in CMV+HIV− persons. The interaction of CD4 on CD4dimCD8bright T cells with major histocompatability complex II (MHC-II) on antigen-presenting cells (APCs) is integral to the increased antigen recognition and functional response of CD4dimCD8bright T cells. These studies show that CD4 expression on CD8+ T cells defines an enriched antiviral cytotoxic population, a critical finding that advances the current paradigm of antiviral cytotoxic lymphocyte (CTL) responses.
Methods
Human protection
This research was conducted in accordance with institutional guidelines on human research and received institutional review board approval from Rush University Medical Center. Donor informed consent was obtained in accordance with the Declaration of Helsinki.
Mononuclear cell subset isolation
Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation from human donor venous blood or obtained from frozen samples. CMV donors were identified by CMV IgG testing (Rush Medical Laboratories). Long-term nonprogressor (LTNP) HIV+ donors were recruited through the Section of Infectious Diseases at Rush University Medical Center.
Immunofluorescence staining and flow cytometric analysis
Surface immunofluorescence staining.
Nonspecific binding of antibodies to cell Fc receptors was blocked using 20 μL/107 cells of FcR blocker (Miltenyi Biotec Inc). Fluorochrome-conjugated antibodies purchased from BD Biosciences PharMingen; Beckman Coulter, Caltag included the following: CD3-fluorescein isothiocyanate (FITC), CD3–Pacific Blue, CD4-phycoerythin (PE), CD4-allophycocyanin (APC*), CD8-FITC, CD8-PE, CD8–peridinin chlorophyll protein (PerCP), CD8-APC*, CD8-APC*-H7, CD8-PerCP-Cy5.5, CD19-PerCP, CD19-APC*-Cy7, CD27-FITC, CD27-APC*, CD28-FITC, CD28-PE, CD38-PE-Cy7, CD45RA-FITC, CD45RA-PE-Cy5, CD45RO-FITC, CD45RO-PE, CD45RO-PE-Cy7, CD57-FITC, CD62L-FITC, CD62L-PE, CD107ab-PE-Cy5, CD107ab-FITC, CCR7-FITC, CCR7-APC*, HLA-A2–FITC, HLA-DR–PerCP, mouse IgG-FITC, mouse IgG-PE, mouse IgG-PerCP, mouse IgG-APC*, A2 CMV pp65-tetramer-PE and APC*, A2 EBV tetramer-PE, A2 HIV Gag-tetramer-PE and APC*, A2 HIV Gag-tetramer-APC, B27 HIV Gag-tetramer-PE, or negative tetramer-PE, Aqua Live/Dead (Invitrogen). Conventional surface immunostaining was performed.
Intracellular cytokine detection assay.
Cells were treated with 10 μg/mL Brefeldin A (Sigma-Aldrich), incubated at 37°C 5% CO2 for up to 6 hours with or without stimulation, and stained for surface markers. Cells were then permeabilized using fluorescence-activated cell sorting Permeabilizing Solution 2 (Becton Dickinson) and immunostained for 30 minutes at 4°C with antibodies to intracellular cytokines. Cells were washed with 1× PBS and fixed in 2% formaldehyde.
TCR clone composition.
Cells were stained ex vivo using the IOTest Beta Mark kit (Beckman Coulter) according to the manufacturer's instructions.
Cytometric analysis.
Four-color and 8-color flow cytometric analyses were performed using a FACSCalibur cytometer with CellQuest (Becton Dickinson) and LSR-II cytometer with Diva software (Becton Dickinson), respectively. Live cells were gated based on negative Aqua Live/Dead staining and lymphocytes based on FSC-A versus SSC-A. When CD3 antibody was used, a gate encompassing CD3+ or CD3+CD8+ cells was created. Next, gates were created encompassing CD8+ populations expressing dim levels of CD4 or not expressing CD4. The percentage of expression of endpoint markers was calculated by the flow analysis software.
Gating on CD4dimCD8bright T cells.
Placement of the CD4dimCD8bright T-cell gate was based on factors visible on a CD4 versus CD8 plot. (1) Beginning point of the gate was based on unstained but compensated samples and samples stained with isotypes (showing endpoint of CD4−CD8+ T-cell population), and (2) endpoint of the gate was based on a line drawn between the endpoint of CD4 staining on monocytes expressing CD4 dimly and the beginning point of CD4 staining on CD4+CD8− T cells expressing CD4 brightly. Sample acquisition was 200 000 to 2 000 000 events.
Immunocytochemistry staining.
PBMCs were stained with CD4-FITC and CD8-PE, as described for flow cytometry, placed on a glass slide, and analyzed using an Olympus Flowview Laser Scanning Microscope (Model-1X81 FVBFUV; Olympus Optical Co) equipped with Fluoview Version 4.3 software. Images were captured using a 20×/0.7 numerical aperture and a 60×/1.4 numerical oil objective.
Lymphocyte activation, priming, and culture
For activation, cells were cultured with 1 μg/mL SEB (Sigma-Aldrich) or 1 μg/mL soluble anti-CD3/anti-CD28 antibodies (PharMingen). Cells from human leukocyte antigen (HLA)–A2*0201+ CMV IgG+ donors were primed with 10 μg/mL of the CMVpp65 peptide NLVPMVATV (Sigma-Aldrich), the immunodominant epitope recognized by CD8+ T cells. Cells from HIV+ donors were primed with pooled HIV peptides (15 amino acids long with 11-amino acid overlaps) spanning the nef, env, gag, and pol regions at 2 μg/peptide/mL (AIDS Research and Reagent Program). HLA typing was performed using Luminex multiplex DNA analysis (Rush University Medical Center Histocompatability and Immunogenetics Laboratory). Unless otherwise noted, cells were cultured in RPMI 1640 medium (BioWhittaker) supplemented with 10% FBS (Gemini Bio-Products) and 1% penicillin/streptomycin (Sigma-Aldrich).
Tetramer assay
Detection of antigen-specific cells was performed using fluorochrome-conjugated CMVpp65 (HLA-A2: NLVPMVATV) or HIVgag (HLA-A2: SLYNTVATL; HLA-B57: KAFSPEVIPMF) MHC-I tetramers. Cells were peptide stimulated or left untreated for 6 days. Cells from CMV+ and HIV+ LTNP donors were primed with CMVpp65 peptide or overlapping HIVgag peptides (4 μg/mL/peptide), respectively, or left untreated. Day 0 fresh and day 6 cells were immunostained for tetramer, CD38, and HLA-DR and analyzed by flow cytometry. Irrelevant (EBV peptide-loaded) and negative (without peptide) MHC-I tetramers were used as controls.
Carboxy fluorescein succinimidyl ester assay
PBMCs were isolated from CMV+ and LTNP HIV+ donors, stimulated with CMVpp65 peptide or HIV pooled peptides, respectively, SEB, or left untreated, and stained with Carboxy fluorescein succinimidyl ester (CFSE; Invitrogen). After a 7-day culture, PBMCs were evaluated by flow cytometry for CFSE fluorescent intensity reduction.
CD107ab cytotoxic potential assay
The CD107ab assay was performed as described by the manufacturer (Becton Dickinson). Briefly, on day 0 effector cells (PBMCs) from CMV+ and HIV+ LNTP donors were isolated and cultured overnight in Aim V serum-free media (Invitrogen). On day 0, T1 and T2 targets (ATCC) were loaded overnight with 40 μg/mL CMVpp65 peptide or HIV pooled peptides at 2 μg/peptide/mL (with the aid of β2-microglobulin; Sigma-Aldrich). Target loading was verified by HLA-A2 up-regulation. On day 1, neutralizing antibodies were added after appropriate stains (ie, CD4 blocker after CD4 stain) to allow for visualization and blocking of the same molecules. Neutralizing antibodies were incubated with appropriate cells for 1 hour and washed. Effectors were stained with CD107ab. Primed effectors and loaded targets were coincubated at 37°C. At 1 hour of coculture, Golgi-block (Becton Dickinson) and Brefeldin A (Sigma-Aldrich) were added to eliminate internalization/degradation of surface CD107ab. The culture was incubated for an additional 4 hours at 37°C. Cells were then immunostained and evaluated by flow cytometry. CD19 was used to distinguish effectors (CD19−) form targets (CD19+). Similar experiments assessed cytotoxic ability of effectors in the presence of CD4-neutralizing antibody (R&D Systems Inc) cultured with effectors or MHC-II blocking antibody (HLA-DP, DQ, DR antibody; Beckton Dickinson) cultured with APCs.
Statistical analyses
Data were analyzed using Prism 4.0 and InStat 3.0 software (GraphPad Software). Two-tailed paired and unpaired t tests were used as indicated. Descriptive statistics and graphical analyses were used where appropriate.
Results
CD4dimCD8bright T cells are a genuine CD8+ T-cell subset
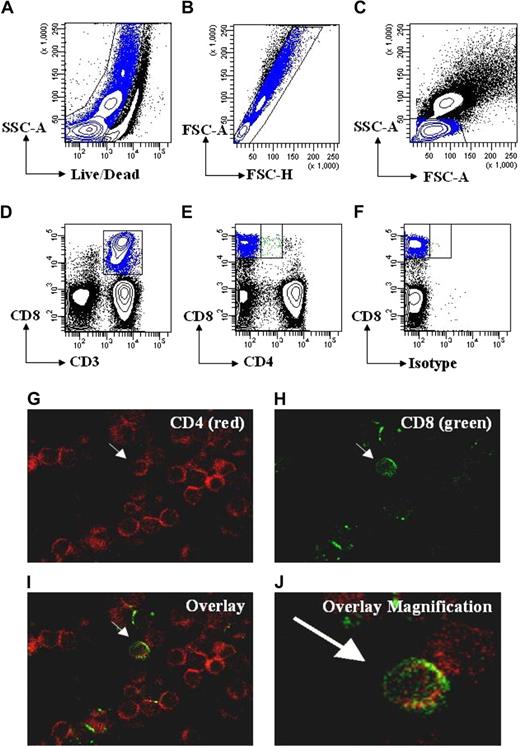
Studies have shown that CD4dimCD8bright T cells represent a genuine, mature, peripheral blood T-cell population.4,5,7-9,12 With advances in multiple parameter flow cytometry we further show that CD4dimCD8bright T cells are live singlet cells and not doublets/debris. We used SSC-A versus Aqua Live/Dead staining and FSC-A versus FSC-H to exclude dead cells (Figure 1A) and doublets (Figure 1B), respectively. We then used FSC-A versus SSC-A to gate on lymphocytes (Figure 1C) and then gated on CD3+CD8+ T cells or all CD3+ T cells (Figure 1D). Finally, we gated on CD4−CD8+ and CD4dimCD8bright T cells (Figure 1E). CD4 staining on CD4dimCD8bright T cells was shown to be not a result of nonspecific antibody binding because (1) panels stained with alternative fluorophore combinations for CD4 and CD8 yielded similar results for CD4dimCD8bright T-cell expression, and (2) CD4dimCD8bright T did not stain with makers not associated with T cells, including CD19, CD56, and CD206 (data not shown). We also visualized these cells using immunocytochemistry staining and confocal microscopy. As seen in Figure 1G-J, CD4dimCD8bright T cells are singlet cells with high (bright) CD8 expression and low (dim) CD4 expression. Taken together, these data lend further evidence that CD4dimCD8bright T cells are a genuine T-cell subpopulation.
CD4dimCD8bright T cells are a genuine subset of CD8+ T cells. (A-D) Gating strategy for live cells using side scatter area (SSC-A) and Aqua Live/Dead staining (A), followed by gating for singlet cells using forward scatter area (FSC-A) and forward scatter height (FSC-H; B), then for lymphocytes using SSC-A and FSC-A(C), and for T cells using CD3 or CD3 and CD8(D). (E) Gating for CD4−CD8+ and CD4dimCD8bright T cells using CD4 and CD8 based on a previous CD3 gate. (F) Representative isotype staining showing gate placement between CD4−CD8+ and CD4dimCD8bright T cells. (G-J) Visualization of CD4dimCD8bright T cells by confocal microscopy. PBMCs were immunostained using anti–CD4-PE (red; G) and anti-CD8-FITC (green; H), then coexpression was visualized by overlay (I). (J) Magnification of overlay in panel I. Arrows point to the same cell coexpressing CD4 and CD8 across the panels.
CD4dimCD8bright T cells are a genuine subset of CD8+ T cells. (A-D) Gating strategy for live cells using side scatter area (SSC-A) and Aqua Live/Dead staining (A), followed by gating for singlet cells using forward scatter area (FSC-A) and forward scatter height (FSC-H; B), then for lymphocytes using SSC-A and FSC-A(C), and for T cells using CD3 or CD3 and CD8(D). (E) Gating for CD4−CD8+ and CD4dimCD8bright T cells using CD4 and CD8 based on a previous CD3 gate. (F) Representative isotype staining showing gate placement between CD4−CD8+ and CD4dimCD8bright T cells. (G-J) Visualization of CD4dimCD8bright T cells by confocal microscopy. PBMCs were immunostained using anti–CD4-PE (red; G) and anti-CD8-FITC (green; H), then coexpression was visualized by overlay (I). (J) Magnification of overlay in panel I. Arrows point to the same cell coexpressing CD4 and CD8 across the panels.
Higher frequency of CD4dimCD8bright T cells among HIV LTNPs
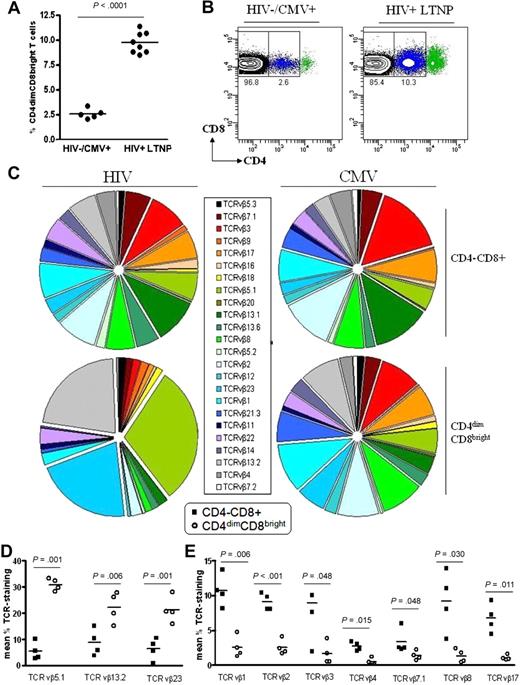
Previous studies from our laboratory have shown that CD4dimCD8bright T cells exhibit higher recognition of CMV-loaded tetramer than do single-positive CD4−CD8+ (SP8) T cells5 ; however, their contribution to anti-HIV immunity is not clear. To evaluate the role of CD4dimCD8bright T cells in anti-HIV immunity, we evaluated their frequency among HIV+ patients who are LTNPs, defined as patients with documented HIV infection for at least 5 years, CD4+ T-cell counts greater than 500 cells/mm3, plasma HIV-1 RNA at low/undetectable levels, and without history of antiretroviral therapy for any extended period. Peripheral blood was obtained from HIV− and LTNP HIV+ donors (Table 1) and evaluated for the percentage of CD4dimCD8bright T-cell expression (Figure 2). Frequency of CD4dimCD8bright T cells was approximately 4-fold higher among HIV+ LTNPs than among HIV− donors (10% versus 2.5%; Figure 2A-B). On the basis of our previous retrospective study of more than 150 chronically infected HIV+ patients whose CD4dimCD8bright T-cell frequency was 0.4% to 3.4%, the frequency of CD4dimCD8bright T cells in HIV+ LTNPs is also consistently higher than in chronically infected HIV+ patients.5 These data show that CD4dimCD8bright T cells are increased in HIV+ LTNPs compared with HIV− and chronically infected HIV+ donors.
Blood donor characteristics
| Donor . | Sex . | Race . | Age, y . | LTNP, y . | CD4, n . | CD4, % . | Viral load . | Treatment . | HLA-A . | HLA-B . | HLA-C . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV+ LTNP | |||||||||||
| HIV 1 | F | AA | 40 | 7 | 1171 | 38 | 470 | AZT (pregnancy in 1994) | 11, 29 | 39, 57 | 06, 07 |
| HIV 1b | F | AA | 44 | 11 | 562 | 27 | 296 | AZT (pregnancy in 1994) | 11, 29 | 39, 57 | 06, 07 |
| HIV 2 | M | H | 42 | 8 | 1172 | 32 | 59 | None | 02, 28 | ND | ND |
| HIV 3 | M | W | 49 | 13 | 1173 | 27 | 1253 | AZT for 2 months in 1994 | 30, 28 | 18, 65 | 08, 08 |
| HIV 4 | F | W | 55 | 12 | 1174 | 43 | <50 | None | 01, 24 | 51, 57 | ND |
| HIV 4b | F | W | 59 | 16 | 749 | 44 | <196 | None | 01, 24 | 51, 57 | ND |
| HIV 5 | M | AA | 45 | 10 | 705 | 29 | 1810 | None | 30, 34 | 14, 42 | 08, 17 |
| HIV 6 | F | AA | 38 | 15 | 553 | 33 | <50 | None | 11, 28 | 51, 58 | 10, 14 |
| HIV 7 | M | W | 47 | 5 | 845 | 39 | 184 | None | 02, 33 | 07, 65 | 07, 08 |
| HIV 8 | M | AA | 28 | 8 | 507 | 32 | <50 | None | 02, 03 | 07, 49 | ND |
| HIV− CMV IgG+ | |||||||||||
| CMV 1 | M | W | 56 | — | — | — | — | — | 02, 02 | 44, 57 | 05, 06 |
| CMV 2 | M | W | 55 | — | — | — | — | — | 02, 03 | 07, 49 | ND |
| CMV 3 | F | W | 44 | — | — | — | — | — | 01, 02 | 08, 44 | 04, 07 |
| CMV 4 | F | H | 44 | — | — | — | — | — | 02, 03 | 35, 51 | 04, 04 |
| CMV 5 | M | W | 55 | — | — | — | — | — | 02, 11 | 38, 51 | 03, 03 |
| Donor . | Sex . | Race . | Age, y . | LTNP, y . | CD4, n . | CD4, % . | Viral load . | Treatment . | HLA-A . | HLA-B . | HLA-C . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV+ LTNP | |||||||||||
| HIV 1 | F | AA | 40 | 7 | 1171 | 38 | 470 | AZT (pregnancy in 1994) | 11, 29 | 39, 57 | 06, 07 |
| HIV 1b | F | AA | 44 | 11 | 562 | 27 | 296 | AZT (pregnancy in 1994) | 11, 29 | 39, 57 | 06, 07 |
| HIV 2 | M | H | 42 | 8 | 1172 | 32 | 59 | None | 02, 28 | ND | ND |
| HIV 3 | M | W | 49 | 13 | 1173 | 27 | 1253 | AZT for 2 months in 1994 | 30, 28 | 18, 65 | 08, 08 |
| HIV 4 | F | W | 55 | 12 | 1174 | 43 | <50 | None | 01, 24 | 51, 57 | ND |
| HIV 4b | F | W | 59 | 16 | 749 | 44 | <196 | None | 01, 24 | 51, 57 | ND |
| HIV 5 | M | AA | 45 | 10 | 705 | 29 | 1810 | None | 30, 34 | 14, 42 | 08, 17 |
| HIV 6 | F | AA | 38 | 15 | 553 | 33 | <50 | None | 11, 28 | 51, 58 | 10, 14 |
| HIV 7 | M | W | 47 | 5 | 845 | 39 | 184 | None | 02, 33 | 07, 65 | 07, 08 |
| HIV 8 | M | AA | 28 | 8 | 507 | 32 | <50 | None | 02, 03 | 07, 49 | ND |
| HIV− CMV IgG+ | |||||||||||
| CMV 1 | M | W | 56 | — | — | — | — | — | 02, 02 | 44, 57 | 05, 06 |
| CMV 2 | M | W | 55 | — | — | — | — | — | 02, 03 | 07, 49 | ND |
| CMV 3 | F | W | 44 | — | — | — | — | — | 01, 02 | 08, 44 | 04, 07 |
| CMV 4 | F | H | 44 | — | — | — | — | — | 02, 03 | 35, 51 | 04, 04 |
| CMV 5 | M | W | 55 | — | — | — | — | — | 02, 11 | 38, 51 | 03, 03 |
LTNP indicates long-term nonprogressor; HLA, human leukocyte antigen; CMV, cytomegalovirus; AA, African American; AZT, azidothymidine; ND, not determined; W, white; and H, Hispanic.
Percentage of expression of CD4dimCD8bright T cells is higher among LTNPs than among HIV seronegative donors. (A-B) Cumulative data (A) and representative dot plots (B) showing CD4 expression on CD8+ T cells obtained from 5 HIV− and 8 HIV+ LTNP donors. (A) Horizontal lines represent the mean percentage of CD4dimCD8bright T cells within the CD8+ T-cell population. (C) Cumulative data showing TCRVβ clonotype expression of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP and CMV+ donors. (D-E) Cumulative data showing TCR vβ clonotype expression of HIV+ LTNPs which are significantly increased (D) or decreased (E) between CD4−CD8+ and CD4dimCD8bright T cells. Closed squares denote CD4−CD8+ T cells and open circles denote CD4dimCD8bright T cells from HIV+ LTNP donors. P values between groups are indicated within the figure and based on 2-tailed unpaired t test analysis.
Percentage of expression of CD4dimCD8bright T cells is higher among LTNPs than among HIV seronegative donors. (A-B) Cumulative data (A) and representative dot plots (B) showing CD4 expression on CD8+ T cells obtained from 5 HIV− and 8 HIV+ LTNP donors. (A) Horizontal lines represent the mean percentage of CD4dimCD8bright T cells within the CD8+ T-cell population. (C) Cumulative data showing TCRVβ clonotype expression of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP and CMV+ donors. (D-E) Cumulative data showing TCR vβ clonotype expression of HIV+ LTNPs which are significantly increased (D) or decreased (E) between CD4−CD8+ and CD4dimCD8bright T cells. Closed squares denote CD4−CD8+ T cells and open circles denote CD4dimCD8bright T cells from HIV+ LTNP donors. P values between groups are indicated within the figure and based on 2-tailed unpaired t test analysis.
Preferential TCRVβ repertoire expression by CD4dimCD8bright T cells of HIV+ LTNPs
To further characterize CD4dimCD8bright T cells and determine whether this population constitutes an invariant T-cell subset, ex vivo PBMCs from HIV+ LTNPs and CMV donors were stained for a set of 24 TCR vβ clonotypes (Beckman Coulter). Although CD4dimCD8bright and CD4−CD8+ T cells from all donors stained for most of the 24 clonotypes, 3 clonotypes (vβ5.1, 13.2, 23) in CD4dimCD8bright T cells of HIV+ LTNPs were significantly increased compared with CD4−CD8+ T cells. These 3 clonotypes constitute greater than 74% of the vβ repertoire of CD4dimCD8bright T cells in HIV+ LTNPs but less than 21% of CD4−CD8+ T cells (Figure 2C-D). Further, 7 clonotypes (vβ1, 2, 3, 4. 7.1, 8, 17) in CD4dimCD8bright T cells of HIV+ LTNPs were significantly decreased in comparison to CD4−CD8+ T cells (Figure 2C,E). The preferential expression of these clonotypes was not seen within CD4dimCD8bright T cells of CMV+ donors, possibly because of differences in viral control between HIV and CMV. These data show a dominant expression of TCRVβ repertoire in CD4dimCD8bright T cells of HIV+ LTNPs, which is not observed within their CD4−CD8+ T-cell counterparts nor among CMV+ donors.
CD4 expression on CD8+ T cells defines a potent anti-HIV cytotoxic CD8+ T-cell population
Because of the higher frequency of CD4dimCD8bright T cells among LTNPs and because of the dysregulation of CD4+ and CD8+ T cells in chronically infected HIV patients, we used blood from LTNPs throughout our functional studies to determine the relevance of this population in mounting anti-HIV responses. We used several complementary approaches to evaluate anti-HIV responses, including evaluation of HIV peptide recognition, polyfunctional cytokine production, and cytotoxic potential response to pooled HIV peptide stimulation. Parallel studies were conducted to evaluate anti-CMV responses in CMV IgG-positive HIV− donors.
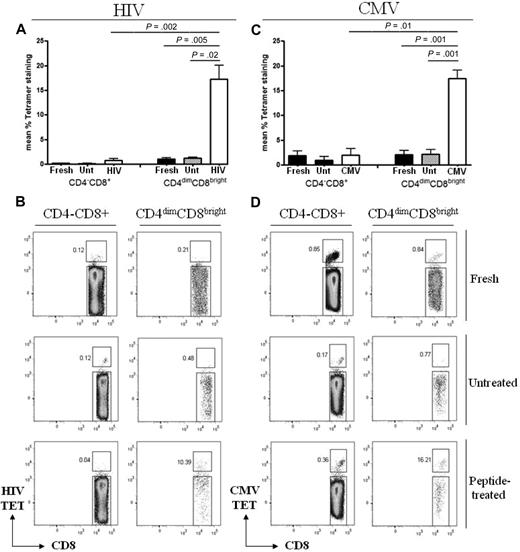
To evaluate antigen recognition by CD4dimCD8bright T cells in comparison to CD4−CD8+ T cells, PBMCs from the respective donors were stimulated with CMVpp65, HIV pooled peptides, or left unstimulated for 6 days then immunostained with HIVgag-loaded, CMVpp65-loaded, irrelevant (EBV), or negative (peptide-free) tetramers. All donors used were HLA typed to ensure compatibility for tetramer staining and target loading (Table 1). After peptide stimulation, respective CD4dimCD8bright T cells bound HIVgag or CMVpp65 tetramer more than 15- and 9-fold higher, respectively, than did CD4−CD8+ T cells (Figure 3). Interestingly, although tetramer recognition by CD4dimCD8bright T cells increased with respective peptide stimulation (compared with no stimulation for the same amount of time), no increase in CD4 expression (percentage or fluorescent intensity) was visualized (data not shown).
CD4dimCD8bright T cells recognize antigen more potently than CD4−CD8+ T cells. (A,C) Cumulative data of CD4−CD8+ and CD4dimCD8bright T cells staining for HIVgag (A) and CMVpp65 (C) tetramers from fresh cells (fresh), untreated cells at day 6 (Unt), or cells stimulated for 6 days with HIV pooled peptide (HIV) or CMVpp65 peptide (CMV) from HIV+ LTNP (A) or CMV+ donors (C). Data represent mean ± SD percentage of expression of tetramer-positive cells from at least 3 donors. P values between groups are indicated within the figure and based on 2-tailed unpaired t test analysis. (B,D) Representative dot plots of HIVgag (B) and CMVpp65 (D) tetramer binding.
CD4dimCD8bright T cells recognize antigen more potently than CD4−CD8+ T cells. (A,C) Cumulative data of CD4−CD8+ and CD4dimCD8bright T cells staining for HIVgag (A) and CMVpp65 (C) tetramers from fresh cells (fresh), untreated cells at day 6 (Unt), or cells stimulated for 6 days with HIV pooled peptide (HIV) or CMVpp65 peptide (CMV) from HIV+ LTNP (A) or CMV+ donors (C). Data represent mean ± SD percentage of expression of tetramer-positive cells from at least 3 donors. P values between groups are indicated within the figure and based on 2-tailed unpaired t test analysis. (B,D) Representative dot plots of HIVgag (B) and CMVpp65 (D) tetramer binding.
On the basis of HLA-DR and CD38 coexpression, CD4dimCD8bright T cells from HIV+ LTNP and CMV+ donors are more activated than are CD4−CD8+ T cells after respective peptide stimulation (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Likewise in the context of tetramer-positive cells, CD4dimCD8bright T cells from HIV+ LTNPs are also more activated than CD4−CD8+ T cells. There was, however, no significant difference between activation status of tetramer-positive CD4dimCD8bright and CD4−CD8+ T cells after CMV peptide stimulation, possibly because CMV stimulation relied on a single epitope (pp65), whereas HIV stimulation was based on pooled peptides.
Given that tetramer binding shows antigen recognition that does not always translate to cytotoxic response,23 we used surface lysosome-associated membrane protein (CD107ab) expression as a marker of cytotoxic potential.24 CD107ab translocates to the cell surface in response to activation-induced degranulation.24 PBMCs from LTNP HIV+ and CMV donors were stained with anti-CD107ab antibodies and cocultured with targets (T1 cells) preloaded with pooled HIV (2 μg/peptide/mL) or CMVpp65 peptides. T1 cells are T:B cell hybrids that express both MHC-I (HLA-A2) and MHC-II (HLA-DR7) and are used here as targets/APCs. PBMCs were cultured alone or with unloaded targets, and background CD107ab expression from CD4−CD8+ and CD4dimCD8bright T cells was used to create a cutoff for CD107ab positivity. Expression of CD107ab was 13-fold and 29-fold higher, respectively, in CD4dimCD8bright T cells than in CD4−CD8+ T cells in response to HIV-loaded and CMV-loaded targets (Figure 4). This higher cytotoxic potential of CD4dimCD8bright T cells was shown at all peptide concentrations used for HIV and CMV-loading of T1 targets (data not shown). These data indicate that CD4dimCD8bright T cells exert a greater cytotoxic potential against CMV and HIV antigens than CD4−CD8+ T cells.
CD4dimCD8bright T cells have greater cytotoxic potential than CD4−CD8+ T cells. (A-B,D-E) Cumulative data (A,D) and representative dot plots (B,E) of CD107ab staining by CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (A-B) and CMV+ (D-E) donors, cocultured with unloaded (left side) or loaded (right side) T1 target cells. (A,D) Data represent mean + SD percentage of 107ab expression from at least 3 donors. P values between groups are indicated within the figure and based on 2-tailed unpaired t test analysis. (C,F) Representative dot plots of CD4−CD8+ (left) and CD4dimCD8bright T cells (right) from an unstained HIV+ LTNP donor (C) and an isotype-stained CMV+ donor (F).
CD4dimCD8bright T cells have greater cytotoxic potential than CD4−CD8+ T cells. (A-B,D-E) Cumulative data (A,D) and representative dot plots (B,E) of CD107ab staining by CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (A-B) and CMV+ (D-E) donors, cocultured with unloaded (left side) or loaded (right side) T1 target cells. (A,D) Data represent mean + SD percentage of 107ab expression from at least 3 donors. P values between groups are indicated within the figure and based on 2-tailed unpaired t test analysis. (C,F) Representative dot plots of CD4−CD8+ (left) and CD4dimCD8bright T cells (right) from an unstained HIV+ LTNP donor (C) and an isotype-stained CMV+ donor (F).
CD4 and MHC-II are required for the enhanced cytotoxic activity of CD4dimCD8bright T cells
In addition to the interaction between the TCR of T cells and MHC molecules of APCs, CD4 on helper T cells and CD8 on cytotoxic T cells, function as a coreceptor by binding to MHC-II or MHC-I, respectively. This binding leads to enhanced T-cell recognition of cognate antigen by enhancing the avidity of the T cell–APC interaction and increasing T-cell signaling. Given that CD4dimCD8bright T cells express both CD4 and CD8 on the same cell, we evaluated if CD4–MHC-II interaction contributes to their potent cytotoxic potential by neutralizing CD4 or MHC-II in the CD107ab assay described above. In our studies, T1 targets were loaded with either CMVpp65 or pooled HIV peptides and treated with MHC-II blocking antibody or left untreated. These cells were then cocultured with PBMCs that were treated with CD4 neutralizing antibody or left untreated. Five hours after coculture of targets and effectors, cells were evaluated for CD107ab expression. Because T1 and T2 cells are a T:B lymphoblastic hybrid, CD19 staining was used to exclude targets in the determination of CD107ab expression by effectors.
Neutralization of the CD4 or MHC-II molecule dramatically diminished the cytotoxic potential of CD4dimCD8bright T cells in response to CMV-loaded and HIV-loaded targets (Figure 5). In CD4dimCD8bright T cells, CD107ab expression in response to HIV antigen-loaded T1 targets was reduced by 85% (from 8.0% to 1.3%) after CD4 neutralization and by 74% (from 8.0% to 2.2%) after treatment with a MHC-II blocker (Figure 5A) Similarly, CD107ab expression in response to CMV-loaded T1 targets was reduced by 57% (from 10.9% to 4.7%) after CD4 neutralization and by 56% (from 10.9% to 4.8%) after MHC-II blocking (Figure 5F). No significant decrease in CD107ab expression was measured in CD4−CD8+ T cells presented with antigen (CMV or HIV) by T1 targets after blocking with MHC-II or CD4 antibodies (Figure 5A,F).
The CD4 molecule contributes to the greater cytotoxic potential of CD4dimCD8bright T cells compared with CD4−CD8+ T cells. (A-H) Cumulative data (A,F) and representative dot plots (B-E,G-J) of CD107ab staining by CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (A-E) and CMV+ (F-J) donors, cocultured with T1 target cells with no blocker (A-B,F-G), after blocking targets with MHC-II blocking antibody(A,C,F,H), or CD4 neutralizing antibody (A,D,F,I) or cocultured with T2 targets (A,E,F,J). (A,F) Data represent mean + SD percentage of 107ab expression from at least 3 donors. P values between groups are indicated within the figure and based on 2-tailed unpaired t test analysis.
The CD4 molecule contributes to the greater cytotoxic potential of CD4dimCD8bright T cells compared with CD4−CD8+ T cells. (A-H) Cumulative data (A,F) and representative dot plots (B-E,G-J) of CD107ab staining by CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (A-E) and CMV+ (F-J) donors, cocultured with T1 target cells with no blocker (A-B,F-G), after blocking targets with MHC-II blocking antibody(A,C,F,H), or CD4 neutralizing antibody (A,D,F,I) or cocultured with T2 targets (A,E,F,J). (A,F) Data represent mean + SD percentage of 107ab expression from at least 3 donors. P values between groups are indicated within the figure and based on 2-tailed unpaired t test analysis.
To further evaluate the contribution of the CD4–MHC-II interaction to the effector function of CD4dimCD8bright T cells, their cytotoxic potential was compared when cocultured with CMV or pooled HIV peptide-loaded targets that express MHC-II (T1 targets) or that do not express MHC-II (T2 targets).25 CD107ab expression by CD4dimCD8bright T cells was reduced by 80% (from 8.6% to 1.6%) when HIV peptide–loaded targets lacked MHC-II (Figure 5A). Similarly, when CMV-loaded T2 targets were cocultured with effector cells, the CD107ab expression by CD4dimCD8bright T cells was reduced by 66% (from 10.9% to 3.7%; Figure 5F). Treatment with neither MHC-II nor CD4 neutralizing antibody had any significant affect on CD4dimCD8bright T cells or CD4−CD8+ T cells presented with antigen by T2 targets (data not shown). These data show that the CD4 molecule on CD4dimCD8bright T cells and MHC-II on APCs plays a major role in enhancing the cytotoxic potential of CD4dimCD8bright T cells.
CD4dimCD8bright T cells are highly enriched for HIV and CMV-specific responses
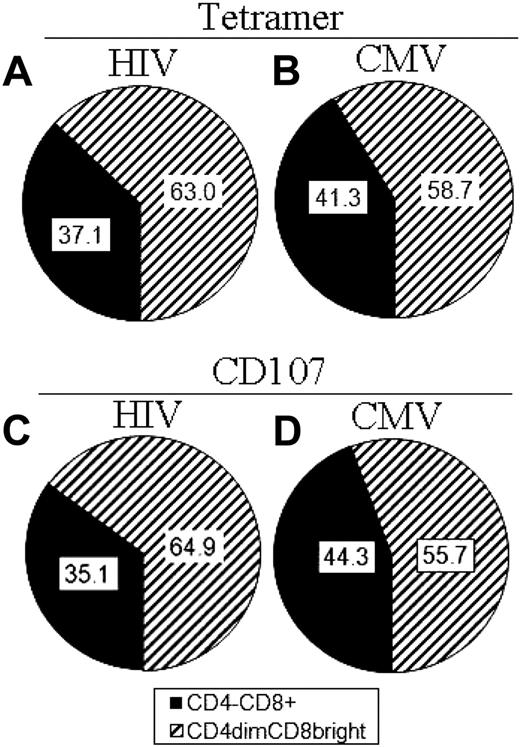
Given that CD4dimCD8bright T cells are less than 3% of CD8+ T cells but generate considerable antigen recognition (Figure 3) and cytotoxic potential (Figures 4–5), we determined the contribution of CD4dimCD8bright T cells to the overall antigen-specific response of CD8+ T cells. We initially gated on all CMV or HIV tetramer+ cells or all CD107ab+ cells and then determined the percentage of these that are CD4dimCD8bright or CD4−CD8+ T cells. Strikingly CD4dimCD8bright T cells constituted greater than 58% of CD8+ tetramer-recognizing T cells (Figure 6A-B) and greater than 55% of CD107ab+ cells (Figure 6C-D) in the context of both HIV and CMV, respectively, after antigen presentation. These dramatic data show that, although CD4dimCD8bright T cells constitute less than 5% of the CD8+ T-cell population, they are highly enriched for antiviral specific responses.
CD4dimCD8bright T cells are highly enriched for antigen-specific responses in comparison to CD4−CD8+ T cells. (A-D) Cumulative data showing percentage of total tetramer binding (antigen recognition; A-B) and total CD107ab staining (cytotoxic potential; C-D) of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (left) and CMV+ (right) donors. Data are based on at least 3 donors.
CD4dimCD8bright T cells are highly enriched for antigen-specific responses in comparison to CD4−CD8+ T cells. (A-D) Cumulative data showing percentage of total tetramer binding (antigen recognition; A-B) and total CD107ab staining (cytotoxic potential; C-D) of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (left) and CMV+ (right) donors. Data are based on at least 3 donors.
CD4dimCD8bright T cells have significantly higher polyfunctional responses than CD4−CD8+ T cells
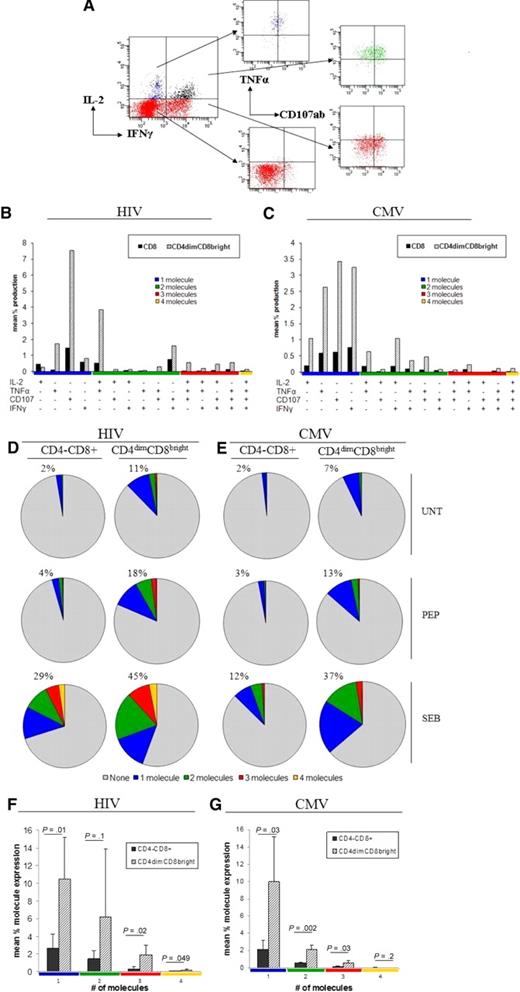
Recent evidence suggests that potent effector responses are mediated by polyfunctional T cells capable of secreting multiple effector molecules.26,27 Retention of polyfunctional T cells is strongly correlated with increased capacity for proliferation and viral control.28 Given that CD4dimCD8bright T cells generate most of the effector responses to HIV and CMV, we determined whether these cells are polyfunctional in response to antigenic challenge. PBMCs from HIV+ LTNP or CMV+ donors were cultured with HIV pooled peptides or CMVpp65, respectively, for up to 6 hours or left untreated, stained with appropriate surface antibodies, permeabilized, and stained with antibodies to intracellular IL-2, IFNγ, TNFα, and surface CD107ab. CD4dimCD8bright T cells produced considerable levels of cytokines and CD107ab either alone or in combination in response to HIV (Figure 7A-B) and CMV (Figure 7C) stimulation. The percentage of expression of total effector molecules (cytokines and CD107ab) was 4-fold higher in CD4dimCD8bright T cells in response to CMV or HIV peptide stimulation and at least 2-fold higher in response to SEB simulation than CD4−CD8+ T cells (Figure 7D-E). Further, significantly higher levels of 1, 3, and 4 molecules per cell were produced by CD4dimCD8bright than by CD4−CD8+ T cells in response to HIV stimulation (Figure 7F) and significantly higher levels of 1, 2, and 3 molecules per cell were produced by CD4dimCD8bright than by CD4−CD8+ T cells in response to CMV stimulation (Figure 7G). These data show that CD4dimCD8bright T cells have significantly higher polyfunctional responses than CD4−CD8+ T cells.
CD4dimCD8bright T cells are the polyfunctional CD8+ T-cell population. (A) Representative dot plots for evaluation of polyfunctional responses. The gating strategy in Figure 1A through F was used to arrive at the cumulative data shown here. (B-C) Cumulative data showing effector molecule production on respective antigen priming of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (B) and CMV+ (C) donors. The x-axis lists the effector molecule or combination produced (production is denoted by +). Responses are grouped and color-coded by the number of effector molecules produced. (D-E) Cumulative data showing the mean percentage of cells producing the respective number of effector molecules with no treatment (UNT), respective peptide treatment (PEP) or SEB treatment. Percentages listed denote total percentage of cells producing one or more molecules (sum of blue, red, green, and orange pies). (F-G) Cumulative data of mean percentage of expression of 1, 2, 3, or 4 molecules in response to HIV(F) or CMV (G) peptide stimulation. P values denote differences between CD4−CD8+ and CD4dimCD8bright T-cell cytokine expression as calculated using paired t test analysis.
CD4dimCD8bright T cells are the polyfunctional CD8+ T-cell population. (A) Representative dot plots for evaluation of polyfunctional responses. The gating strategy in Figure 1A through F was used to arrive at the cumulative data shown here. (B-C) Cumulative data showing effector molecule production on respective antigen priming of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (B) and CMV+ (C) donors. The x-axis lists the effector molecule or combination produced (production is denoted by +). Responses are grouped and color-coded by the number of effector molecules produced. (D-E) Cumulative data showing the mean percentage of cells producing the respective number of effector molecules with no treatment (UNT), respective peptide treatment (PEP) or SEB treatment. Percentages listed denote total percentage of cells producing one or more molecules (sum of blue, red, green, and orange pies). (F-G) Cumulative data of mean percentage of expression of 1, 2, 3, or 4 molecules in response to HIV(F) or CMV (G) peptide stimulation. P values denote differences between CD4−CD8+ and CD4dimCD8bright T-cell cytokine expression as calculated using paired t test analysis.
CD4dimCD8bright T cells are central memory T cells
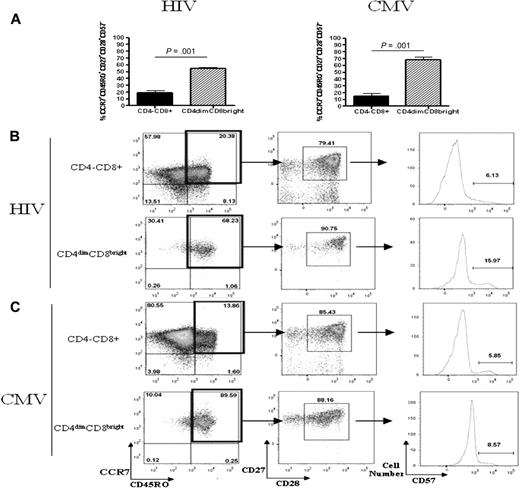
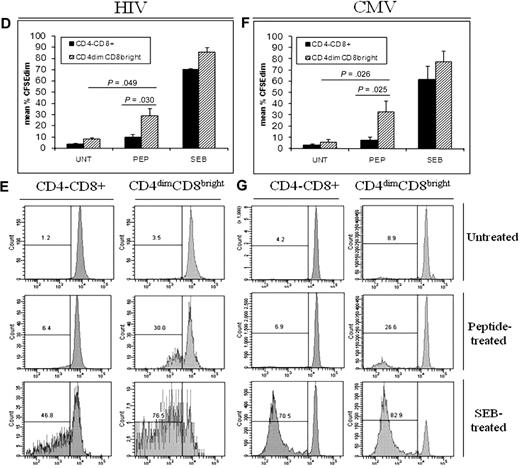
Current knowledge of CD8+ T-cell differentiation indicates that at least 3 memory subsets exist: central memory, effector memory, and terminally differentiated memory. These subsets can be distinguished by surface marker expression and proliferation capability (as reviewed by Sallusto et al29 ). On the basis of surface maker staining, most CD4dimCD8bright T cells from both HIV and CMV donors were CCR7+CD45RO+CD27+CD28+ and CD57−, identifying them as central memory T cells (Figure 8A). CD4−CD8+ T cells were more evenly distributed among the numerous memory subsets (Figure 8B-C). CD4dimCD8bright T cells also proliferated to a greater extent in response to HIV and CMV peptide stimulation than did CD4−CD8+ T cells (Figure 8D,F). Specifically, PBMCs were stained with CFSE and cultured for 7 days with HIV pooled peptides, CMVpp65, SEB, or left untreated and stained with surface antibodies to relevant markers. CD4dimCD8bright T cells proliferated at a rate approximately 5-fold and 4-fold higher in response to HIV and CMV peptide stimulation, respectively, than did CD4−CD8+ T cells (Figure 8D,F). Therefore, on the basis of surface marker expression and proliferative capability, most CD4dimCD8bright T cells are central memory cells.
Most CD4dimCD8bright T cells are central memory T cells. (A) Cumulative data showing mean percentage of CD4−CD8+ (left) and CD4dimCD8bright (right) T cells, from HIV+ LTNP (A-B) and CMV+ (A,C) donors, that are central memory (CCR7+CD45RO+CD27+CD28+CD57−). (B-C) Representative dot plots showing raw percentages of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (B) and CMV+ (C) donors staining for memory-associated markers CCR7+CD45RO+ (left), then further gated CD27+CD28+ cells, and finally CD57+ cells. (D-G) Cumulative data (D,F) and representative flow dot plots (E,G) showing the proportion of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (D-E) and CMV+ (F-G) donor cells that are CFSEdim (decreased CFSE expression and, therefore, increased proliferation) in response to no treatment (UNT), respective peptide treatment (PEP), or SEB treatment. (A,D,F) Data are based on at least 3 donors + SD. P values between groups are indicated within the figure and based on 2-tailed paired t test analysis.
Most CD4dimCD8bright T cells are central memory T cells. (A) Cumulative data showing mean percentage of CD4−CD8+ (left) and CD4dimCD8bright (right) T cells, from HIV+ LTNP (A-B) and CMV+ (A,C) donors, that are central memory (CCR7+CD45RO+CD27+CD28+CD57−). (B-C) Representative dot plots showing raw percentages of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (B) and CMV+ (C) donors staining for memory-associated markers CCR7+CD45RO+ (left), then further gated CD27+CD28+ cells, and finally CD57+ cells. (D-G) Cumulative data (D,F) and representative flow dot plots (E,G) showing the proportion of CD4−CD8+ and CD4dimCD8bright T cells from HIV+ LTNP (D-E) and CMV+ (F-G) donor cells that are CFSEdim (decreased CFSE expression and, therefore, increased proliferation) in response to no treatment (UNT), respective peptide treatment (PEP), or SEB treatment. (A,D,F) Data are based on at least 3 donors + SD. P values between groups are indicated within the figure and based on 2-tailed paired t test analysis.
Discussion
Despite significant compelling evidence for the existence of CD4dimCD8bright T cells and their potential role in antiviral immune responses,5,10,11 this population is often neglected in the analysis of antiviral CTL responses. CD4dimCD8bright T cells are either gated out from conventional flow cytometric analyses or grouped together with CD4−CD8+ T cells without identification or recognition of their contribution to the overall CD8+ T-cell antiviral response. We demonstrate here that CD4dimCD8bright T cells are highly enriched in antiviral responses in comparison to CD8+ T cells that do not express CD4 on their surface. This finding promotes a new paradigm for the evaluation of CD8+ T-cell antiviral responses. By neglecting this subset, evaluations of antiviral vaccine responses are compromised whereby potential vaccines may be deemed not to elicit potent CTL responses, when in fact they may once CD4dimCD8bright T-cell subsets are included in the analysis.
It is especially striking that CD4dimCD8bright T cells are less than 5% of CD8+ T cells, yet they generate greater than 55% of cytotoxic responses to CMV and HIV antigens. Expression of CD4 on effector cells and MHC-II on APCs is critical for this higher effector response from CD4dimCD8bright T cells. Adoptive transfer of CD8+ T cells from CD4-knockout mice to LCMV-infected mice also results in diminished immune responses.11 These studies highlight a biologic role for CD4 on CD8+ T cells in the enhancement of immunologic responses to viral infections. The exact mechanism by which CD4 exerts this heightened antiviral response on CD8+ T cells is not clear. Given our finding that CD4dimCD8bright T-cell antiviral responses are diminished by blocking CD4 on CD4dimCD8bright T cells or MHC-II on APCs, we propose a “double-arm” model by which CD4dimCD8bright T cells exert a more potent antiviral response. In our model, CD4 on CD4dimCD8bright T cells binds MHC-II on APCs, whereas TCR and CD8 conventionally bind MHC-I:peptide on APCs. This double-arm contact may enhance antigen avidity or signal transduction for CD8+ T-cell activation. Indeed, the CD4 molecule on CD4dimCD8bright T cells is associated with Src-family kinase Lck, which promotes T-cell activation.7 CD4 on CD4dimCD8bright T cells may also enhance their chemotaxis ability, as was shown by the their ability to migrate toward IL-16 (a ligand for CD4) in a CD4-dependent manner.9
CD4dimCD8bright T cells probably represent a distinct subpopulation of CD8+ T cells rather than a transient phenotype of CD8+ T-cell activation. In fact, expression of CD4 on CD8+ T cells may be an early marker for CD8+ T-cell differentiation, especially identifying CD8+ T cells that respond to a cognate antigen. Several findings lend support to this hypothesis. (1) Naive CD8+ T cells up-regulate CD4 to a greater degree than do memory CD8+ T cells,8 and expression of CD4 is stable over time both in vitro and ex vivo.4,5 (2) CD4dimCD8bright T cells are inherently more activated than CD4−CD8+ T cells, because they express elevated levels of classic T-cell activation markers (CD95, CD25, CD38, CD69, CD28, CD45RO) to higher degrees than do CD4−CD8+ T cells.4 In addition, despite their activation state, they are protected from activation-induced cell death.5 (3) Greater antigen recognition and immune response of CD4dimCD8bright T cells compared with CD4−CD8+ T cells are not restricted to HIV and CMV. CD4dimCD8bright T cells also recognized hepatitis C virus more potently than do their SP8 T-cell counterparts.30 (4) We demonstrate here that most of the CD4dimCD8bright T cells are central memory, a long-lived memory subset. Collectively, these findings suggest that CD4 up-regulation on CD8+ T cells is an initial response that tags the CD8+ T cells that first recognize cognate antigen. CD4dimCD8bright T cells then become activated, exert their effector responses, and differentiate into central memory T cells.
Elevated expression of CD4dimCD8bright T cells among LTNPs suggests that they may contribute to the ability of these patients to control HIV replication in the absence of antiretroviral therapy. Conversely, dysregulation or loss of CD4dimCD8bright T cells may be linked to HIV disease progression. Ironically, CD4 expression on CD8+ T cells may also enhance their susceptibility to HIV infection. HIV is detected within a minor population of CD8+ T cells and may be attributed to CD4 expression on these cells.31 However, we have previously demonstrated that CD4dimCD8bright T cells are productively infected by T-tropic and not M-tropic HIV because they secret β-chemokines that sequester CCR5 away from HIV gp120.5 In early HIV disease, as M-tropic HIV predominates, it is probable that CD4dimCD8bright T cells remain viable and contribute to anti-HIV immune responses. In late HIV disease, as T-tropic HIV predominates, CD4dimCD8bright T cells may become infected and dysregulated or lost. Contrary to our findings that CD4dimCD8bright T cells are preferentially infected by T-tropic and not M-tropic HIV, others have shown that they are infected by M-tropic HIV.12 These differences are probably due to the mechanism by which CD4 is induced on CD8+ T cells. Anti-CD3/CD28 costimulation led to potent β-chemokine production and thus resistance to M-tropic HIV infection while anti-CD3 mobilization may have not.8
We previously demonstrated that CD4dimCD8bright T cells generated in vitro through stimulation of CD4-depleted CD8+ T cells with SEB or anti-CD3/CD28 secrete predominately IL-4.5 However, blood CD4dimCD8bright T cells in a recall response (antigen stimulation) secret polyfunctional Th1 and Th2 cytokines. These data indicate that the cytokine profile of these cells may differ depending on the mode of stimulation.
Double-positive cells have been described in the intestine of rhesus macaques32 and were shown to be highly activated and responsive to mitogen stimulation.32 However, it is unclear if these intestinal macaque double-positive cells are the same cells described in our studies. In the intestine, the CD8 molecule can be αα or αβ. CD4dimCD8bright T cells described here are αβ and not αα.4 Further, the analysis of the intestinal macaque cells has included all double-positive cells, including CD4 T cells that express CD8. In our studies, it is the CD4dimCD8bright T cells that exert higher antigen specificity and not the CD4brightCD8bright or CD4+CD8dim T cells. Therefore, contradictory results from various groups may be due to the manner in which the term “double-positive” is defined because several subpopulations of double-positive cells exist based on differences in CD4 and CD8 expression intensity.6
A population that is CD4brightCD8bright T cells also exists. We concentrated on CD4dimCD8bright T cells rather than on CD4brightCD8bright T cells for several reasons. (1) When CD4−CD8+ T cells are stimulated, they up-regulate CD4 dimly on their surface, and further stimulation has not yielded the CD4brightCD8bright population. (2) Previous investigation of CD4brightCD8bright T cells have shown them to not exhibit the effector responses (antigen recognition and cytokine production) attributed to CD4dimCD8bright T cells, which may be due to their status of exhaustion or senescence because they express higher levels of CD57 and PD-1, respectively (data not shown). (3) CD4+CD8− T cells have also been shown to up-regulate CD8 on stimulation; therefore, CD4brightCD8bright T cells may represent a combination of cells derived from CD4+ and CD8+ T cells and not a homogenous population as are CD4dimCD8bright T cells, which are only derived from CD8+ T cells.
Expression of otherwise unconventional molecules on lymphocyte subsets is not unique to CD4dimCD8bright T cells. NK T cells express molecules that are reflective of both NK and T cells. Further, a unique lymphocyte population expressing both phenotypic and functional properties of NK and dendritic cells, namely NK dendritic cells have been shown to exist.33,34 Because the contribution of CD4dimCD8bright T cells to immunity is not limited to anti-HIV responses, as they exert potent responses to CMV, LCMV, HIV, and HCV, understanding the mechanism by which CD4 induces higher CD8+ T-cell responses is beneficial for understanding CD8+ T-cell biology, which can be harnessed for antiviral vaccine strategies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Donald Sodora (Seattle Biomedical Research Institute,) and Joseph Kovacs (NIH Clinical Center, AIDS Section, National Institutes of Health) for critical evaluation of the manuscript. We thank Nick Kannon (Department of Neurosciences, Rush University Medical Center) for assistance with confocal microscopy, and the Jane B. Pendleton Charitable Trust for purchase of the Becton Dickinson LSR II flow cytometer.
This work was supported by a grant from the Campbell Foundation.
Authorship
Contribution: A.Z. designed and performed the experiments, created all of the figures, and wrote the manuscript; J.S. performed the experiments with assistance from J.M. and P.M.J; A.R.T. recruited patients and edited the manuscript; and L.A.-H. designed the experiments and wrote the manuscript. All authors discussed the data and provided comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for P.M.J. is Medical College of Wisconsin, Department of Surgery, 9200 W Wisconsin Ave, Milwaukee, WI 53226.
Correspondence: Lena Al-Harthi, Department of Immunology/Microbiology, Rush University Medical Center, 1735 West Harrison St, 614 Cohn, Chicago, IL 60612; e-mail: lena_al-harthi@rush.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal