Abstract
We present results of peripheral blood stem cell (PBSC) mobilization, collection, and follow-up from 3928 consecutive unrelated stem cell donors. Assessments were performed prospectively at baseline, leukapheresis, 1 month, 6 months, and annually after PBSC donation. During follow-up, side effects were recorded by return post questionnaires. The median CD34+ cell counts on day 5 were 67.5/μL in male and 51/μL in female donors. Bone pain and headache were the most common side effects of recombinant human granulocyte-colony stimulating factor. Central venous access was required for 23 donations (0.6%). Throughout the follow-up, the absolute neutrophil counts were slightly below the initial baseline values but remained within the normal range. The majority of the donors reported good or very good health. Malignancies occurred in 12 donors (0.3%), among whom were 1 case of acute myeloid leukemia, 1 case of chronic lymphatic leukemia, and 2 cases of Hodgkin disease. Only the incidence of Hodgkin lymphoma differed significantly from an age-adjusted population. In conclusion, 7.5 μg/kg per day lenograstim proved to be safe and effective for mobilizing hematopoietic stem cells for allogeneic transplantation. Long-term monitoring of healthy PBSC donors remains important to guarantee the safety standards of PBSC mobilization and collection.
Introduction
The collection of hematopoietic stem cells mobilized from the bone marrow into the bloodstream of healthy donors has now become a routine procedure throughout the world. In Germany, peripheral blood stem cells (PBSCs) mobilized by recombinant human granulocyte-colony stimulating factor (rhG-CSF) were used for 1739 (86.7% of all) allogeneic hematopoietic stem cell transplantations in 2006. The immediate side effects of rhG-CSF administration and PBSC collection have long been established; however, information as to possible long-term consequences has been limited up to now.1 To reduce the risk of complications in healthy donors, the dose of rhG-CSF should be as low as possible. At the same time, it is in the interests of both the donor and recipient to obtain enough stem cells to avoid graft failure. The optimal dose for achieving both these aims has yet to be precisely determined. Our prospective study at the University Hospital of Dresden has evaluated the efficiency and consequences, both short- and long-term, of PBSC mobilization and harvesting in a large population of healthy unrelated donors.
Methods
Subjects
The PBSC mobilization protocol was approved by the Ethics Committee of the University Hospital of Dresden, and donor informed consent was obtained in accordance with the Declaration of Helsinki. We studied 3928 healthy unrelated donors who consecutively donated PBSCs between January 1996 and January 2008. Within this group, 675 donors donated for recipients from the United States, but mobilization results and follow-up data have not been reported to the National Marrow Donor Program. Overall, 2813 men (71.6%) and 1115 women (28.4%), with a median age of 33.9 years (range, 18-61 years), were included in 4050 cycles of rhG-CSF administration and apheresis. In 122 donors (3.1%), a second cycle of rhG-CSF mobilization and PBSC collection was performed in response to a second donation request from the transplantation center or for a different recipient, after central review by the supervising physicians of the donor center. The median interval between the first and second PBSC donations was 265 days (range, 22-510 days).
PBSC mobilization and collection
The standard mobilization regimen, which was used in 97.3% of the donors, was 7.5 μg/kg rhG-CSF (lenograstim) administered for 5 to 6 consecutive days. In some protocols, donors received filgrastim at 10 μg/kg (2%) or PEG-filgrastim as a 12-mg single dose (1%). Experience from mobilization protocols with filgrastim and PEG-filgrastim has been reported elsewhere.2,3 The first leukapheresis was performed on day 5. If the required number of CD34+ cells per recipient body weight was not collected, rhG-CSF administration was repeated (depending on the donor's leukocyte count), and a second PBSC collection was performed on day 6.
The rhG-CSF was injected subcutaneously either daily by the donor's family doctor (15.3%) or every 12 hours by the donor or a family member (81.9%). PBSCs were collected by a continuous-flow blood cell separator (Cobe Spectra; Caridian BCT) via bilateral (anterior cubital and forearm) peripheral venous access, whenever possible, or otherwise via a central line in the femoral vein. In donors who needed a central line, only one leukapheresis was performed and the venous line was removed immediately afterward. During the first leukapheresis, the donor's total blood volume was processed 4 to 5 times at 50 to 110 mL per minute over 3 to 4.5 hours. Anticoagulation was maintained with acid-citrate dextrose A (ratio 1:12-20) and heparin (5000 U/donor). During a second leukapheresis, if necessary, the donor's blood volume was processed only 3 times, and heparin was used only if the platelet count was more than 100 × 109/L. The number of CD34+ cells required from the donor was 4 to 10 × 106/kg of recipient body weight, dependent on the disease and treatment protocol. Two leukaphereses were performed in 892 (22%) donors. Only one donor, a female, underwent 3 leukaphereses because of very poor mobilization.
Monitoring of PBSC donors
Complete blood counts and differentials were performed at the initial consultation (2-4 weeks before donation), then before and after each leukapheresis. During and immediately after the apheresis, vital signs and adverse events were documented by the medical staff. Although spleen size was not routinely monitored during mobilization, abdominal sonography was performed if the spleen had been enlarged at baseline or if the donor had reported abdominal discomfort.
Follow-up investigations
Immediately after the last apheresis, all donors completed a questionnaire as to side effects of rhG-CSF mobilization and apheresis. Subsequently, at 1 month, 6 months, and annually up to 5 years thereafter, the donors received another questionnaire regarding their overall condition and particular symptoms possibly related to rhG-CSF administration and apheresis. Complete blood count, differential counts, and biochemistry results were obtained initially by the Dresden University Hospital laboratory and later by the donor's own doctor. Data were collected in the apheresis unit and at the DKMS German Bone Marrow Donor Center. Donors who reported symptoms or had abnormal laboratory results within the first month were contacted by mail and telephone interview by the apheresis physicians. The follow-up investigations at 6 months and later were organized by the DKMS German Bone Marrow Donor Center.
Data management and statistical analysis
All data were prospectively collected at the apheresis center with the assistance of the DKMS German Bone Marrow Donor Center.
Statistics were analyzed using SPSS software. Wilcoxon test for paired samples was used to estimate significant changes for the respective parameters. The significance level for single tests was set at 5%. Where not indicated otherwise, data shown represent median values and ranges. The incidences of malignancies in our donors were compared with the incidence of each different malignancy in the age-adjusted population, according to the methods described by Estève et al.4 Age adjustment was performed by indirect standardization. Where age-specific data were available, incidence rates for 5-year age groups were applied to the appropriate person-years at risk to compute the expected numbers of malignancies and observed-to-expected ratios, also called standardized incidence ratios (SIR). The exact Poisson distribution was used to calculate 95% confidence intervals.
Because of the explorative character of the analysis, no α adjustment was performed.
Results
Efficacy of PBSC mobilization
On day 5 of rhG-CSF administration, 6 to 285/μL (median 62/μL) CD34+ stem cells had been mobilized into the peripheral blood. There were significantly higher yields in male compared with female donors (P = .000, Figure 1A). The median total CD34+ yield from the first leukapheresis was 5.88 (0.16-27.39) × 108 cells. A single leukapheresis was sufficient to collect the CD34+ cell dose required in 3072 donors (78.2%). In the majority of donors (99.5%), enough CD34+ cells could be collected in 1 or 2 aphereses. In female donors, a second leukapheresis had to be performed more often than in male donors (37.4% vs 15.9%). Only in 18 donors (0.45%) was the CD34+ yield less than 2 × 106/kg recipient body weight (Figure 1B).
Efficacy of CD34+ mobilization and PBSC yield. Estimates of the frequencies of CD34+ cell count in peripheral blood at day 5 of rhG-CSF administration in male and female donors (A) and the cumulative CD34+ yield (×106/kg recipient body weight) in donors with 2 leukaphereses (B).
Efficacy of CD34+ mobilization and PBSC yield. Estimates of the frequencies of CD34+ cell count in peripheral blood at day 5 of rhG-CSF administration in male and female donors (A) and the cumulative CD34+ yield (×106/kg recipient body weight) in donors with 2 leukaphereses (B).
Hematologic parameters
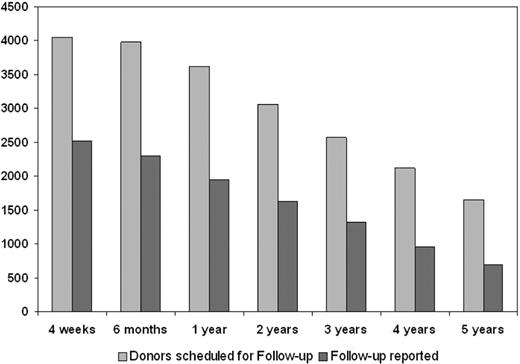
The percentage of donors available for follow-up was 62.1% at 4 weeks and declined thereafter to 42.5% at 5 years after PBSC donation (Figure 2). Altogether, the follow-up data include 8234 donor-years. Hematologic parameters after rhG-CSF administration and in the follow-up period are shown in Table 1. The median white blood cell count (WBC) increased on mobilization from 6.28 to 41.74 × 109/L (P < .001). This increase was the result not only of an increase in neutrophils (to 8.28× baseline values) but also of an increase in lymphocytes (2.28× baseline) and monocytes (10.08× baseline).
Availability of follow-up reports from the donors at various time points. Proportion of donors replying to the follow-up survey is shown by the dark gray bars.
Availability of follow-up reports from the donors at various time points. Proportion of donors replying to the follow-up survey is shown by the dark gray bars.
Hematologic values during rhG-CSF administration, leukapheresis, and follow-up
| Variable . | Baseline . | Before first apheresis . | 30 d . | 6 mo . | 1 y . | 2 y . | 3 y . | 4 y . | 5 y . |
|---|---|---|---|---|---|---|---|---|---|
| WBC, ×109/L | 6.28 (2.93-19.02) | 41.74* (4.64-94.10) | 5.10† (2.20-15.00) | 5.60† (2.31-18.10) | 5.80† (1.90-15.30) | 5.90† (1.59-16.00) | 5.94† (3.1-17.13) | 6.00† (3.09-13.60) | 6.1† (2.20-17.00) |
| Neutrophil count, ×109/L | 3.78 (1.21-14.80) | 31.30* (5.13-87.00) | 2.93† (0.81-12.50) | 3.24† (0.54-15.00) | 3.26† (0.90-12.30) | 3.33† (0.99-12.61) | 3.32† (1.20-12.52) | 3.33† (0.18-9.83) | 3.44† (1.01-12.27) |
| Lymphocyte count, ×109/L | 1.78 (0.29-5.38) | 4.06* (1.02-15.20) | 1.48† (0.44-4.01) | 1.64† (0.44-4.32) | 1.69† (0.61-5.18) | 1.80* (0.12-6.18) | 1.83* (0.64-12.33) | 1.86* (0.61-4.44) | 1.90* (0.68-8.00) |
| Hemoglobin level, mmol/L | 9.20 (5.03-11.50) | 9.00† (6.60-11.00) | 9.06† (6.15-11.67) | 9.20* (6.27-11.36) | 9.25* (6.33-11.42) | 9.19 (6.52-11.30) | 9.12 (5.65-11.24) | 9.12 (6.33-11.30) | 9.19 (6.52-11.11) |
| Platelet count, ×109/L | 245 (66-533) | 224† (71-494) | 235† (72-544) | 244 (82-474) | 245 (83-579) | 244 (58-544) | 247 (77-465) | 245 (111-475) | 245 (98-524) |
| No. of donors | 3934 | 3950 | 2307 | 2203 | 1856 | 1562 | 1257 | 916 | 659 |
| Variable . | Baseline . | Before first apheresis . | 30 d . | 6 mo . | 1 y . | 2 y . | 3 y . | 4 y . | 5 y . |
|---|---|---|---|---|---|---|---|---|---|
| WBC, ×109/L | 6.28 (2.93-19.02) | 41.74* (4.64-94.10) | 5.10† (2.20-15.00) | 5.60† (2.31-18.10) | 5.80† (1.90-15.30) | 5.90† (1.59-16.00) | 5.94† (3.1-17.13) | 6.00† (3.09-13.60) | 6.1† (2.20-17.00) |
| Neutrophil count, ×109/L | 3.78 (1.21-14.80) | 31.30* (5.13-87.00) | 2.93† (0.81-12.50) | 3.24† (0.54-15.00) | 3.26† (0.90-12.30) | 3.33† (0.99-12.61) | 3.32† (1.20-12.52) | 3.33† (0.18-9.83) | 3.44† (1.01-12.27) |
| Lymphocyte count, ×109/L | 1.78 (0.29-5.38) | 4.06* (1.02-15.20) | 1.48† (0.44-4.01) | 1.64† (0.44-4.32) | 1.69† (0.61-5.18) | 1.80* (0.12-6.18) | 1.83* (0.64-12.33) | 1.86* (0.61-4.44) | 1.90* (0.68-8.00) |
| Hemoglobin level, mmol/L | 9.20 (5.03-11.50) | 9.00† (6.60-11.00) | 9.06† (6.15-11.67) | 9.20* (6.27-11.36) | 9.25* (6.33-11.42) | 9.19 (6.52-11.30) | 9.12 (5.65-11.24) | 9.12 (6.33-11.30) | 9.19 (6.52-11.11) |
| Platelet count, ×109/L | 245 (66-533) | 224† (71-494) | 235† (72-544) | 244 (82-474) | 245 (83-579) | 244 (58-544) | 247 (77-465) | 245 (111-475) | 245 (98-524) |
| No. of donors | 3934 | 3950 | 2307 | 2203 | 1856 | 1562 | 1257 | 916 | 659 |
Values are median (range).
P < .001, higher than baseline.
P < .001, lower than baseline.
There was a slight, but significant, decrease of platelet counts and hemoglobin levels after rhG-CSF administration, which persisted 30 days after PBSC donation. Leukocyte counts 4 weeks after PBSC collection were significantly lower than the WBC on day 0 (P < .001). After 6 months, 1 year, and 5 years after PBSC donation, the WBCs were higher than at 4 weeks but never completely returned to baseline values. Changes in absolute neutrophil counts (ANCs) resembled those in WBC. Lymphocyte counts were significantly diminished up to 1 year after PBSC donation and were slightly elevated after 2 to 5 years. Platelet counts reached pretreatment values 6 months after apheresis and remained stable thereafter.
Side effects of rhG-CSF administration and leukapheresis
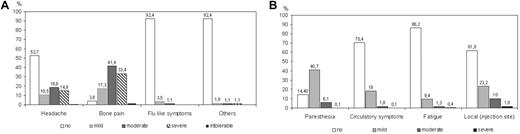
Complaints of the donors during PBSC mobilization are shown in Figure 3A. The predominant side effect of rhG-CSF administration was bone pain, reported in 93.5% of the donors. Headache occurred in 34%. Flu-like symptoms and other symptoms altogether appeared in 8.7% of the donor population. There was no case of splenic rupture in the donors investigated. No donor discontinued rhG-CSF administration because of side effects. One donor was hospitalized with pneumonia during rhG-CSF administration but completed PBSC donation successfully.
Side effects of rhG-CSF application and leukapheresis. Estimates of the percentage of donors reporting side effects during the period of rhG-CSF administration (A) and during leukapheresis (B).
Side effects of rhG-CSF application and leukapheresis. Estimates of the percentage of donors reporting side effects during the period of rhG-CSF administration (A) and during leukapheresis (B).
The most frequent symptom during leukapheresis was paresthesia associated with hypocalcemia. Paresthesia was mild or moderate in most (46.8%) donors and quickly disappeared after intravenous or oral calcium substitution. Other problems, such as circulatory disturbances, fatigue, or pain at the site of venipuncture, occurred infrequently (Figure 3B). Access via the femoral vein was required in 23 donations only (0.6%), and in none of these donors did the central line cause complications. Because of low platelet counts after the second apheresis, autologous platelet retransfusion was performed in 248 (5%) of the PBSC collections.
Adverse events during follow-up
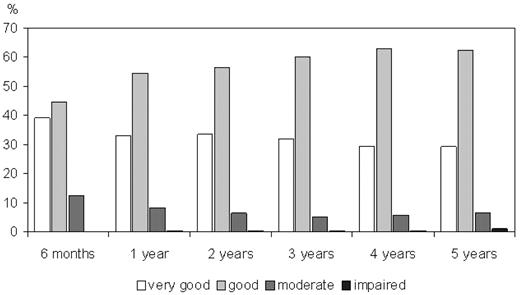
General health status and adverse events in the donors during 5 years after the PBSC collection are shown in Figure 4 and Table 2. Most of the donors reported good or very good general health. Infections were reported by 1.0% to 2.9%, and headache by 1.0% to 1.8% of the donors during the follow-up period. In 10% to 18% of the donors, some impairment of well-being was reported. Serious symptoms (resulting in hospitalization) occurred only in less than or equal to 1.1% of the donor population. Among these donors, 12 persons (0.30% of the whole group) developed malignancies (Table 3). This follow-up includes 8234 donor-years. Where data regarding age-related incidences in the German population were available, standardized incidence ratios and confidence intervals were computed (Table 3). In the case of chronic lymphocytic leukemia (CLL), the age-related incidence in Germany has not been ascertained yet; therefore, US data were used as controls.10 In very rare tumors (neuroendocrine carcinoma and brain tumor), no age-related incidences were available; therefore, no statistical analysis could be performed. Regarding the incidences of hematologic malignancies, which were acute myeloid leukemia (AML), CLL, and Hodgkin lymphoma, a significant deviation from the expected rate in an age-adjusted control population was detected only for Hodgkin disease (standardized incidence ratio = 8.86; 95% confidence interval, 1.06-31.98).
General health status of donors during the follow-up period. Estimates of overall condition (percentage of all donors responding to the follow-up survey). Estimates are self-assessments of the donors.
General health status of donors during the follow-up period. Estimates of overall condition (percentage of all donors responding to the follow-up survey). Estimates are self-assessments of the donors.
Donor complaints during the follow-up period (time after donation)
| Complaint . | 6 mo . | 1 y . | 2 y . | 3 y . | 4 y . | 5 y . |
|---|---|---|---|---|---|---|
| Bleeding tendency | ||||||
| Yes | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 | 0.3 |
| No | 95.7 | 96.1 | 96.7 | 97.4 | 98.3 | 98.9 |
| Infections | ||||||
| Yes | 2.9 | 2.4 | 1.9 | 2.0 | 2.0 | 1.0 |
| No | 93.0 | 93.8 | 94,9 | 95.5 | 96.3 | 97.5 |
| Headache | ||||||
| Yes | 1.0 | 1.3 | 0.9 | 1.4 | 1.4 | 1.8 |
| No | 95.0 | 94.8 | 95.9 | 96.2 | 96.8 | 97.4 |
| Bone pain | ||||||
| Yes | 0.7 | 0.9 | 0.5 | 0.4 | 0.4 | 0.8 |
| No | 95.2 | 95.3 | 96.3 | 97.2 | 97.9 | 98.3 |
| Complaint . | 6 mo . | 1 y . | 2 y . | 3 y . | 4 y . | 5 y . |
|---|---|---|---|---|---|---|
| Bleeding tendency | ||||||
| Yes | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 | 0.3 |
| No | 95.7 | 96.1 | 96.7 | 97.4 | 98.3 | 98.9 |
| Infections | ||||||
| Yes | 2.9 | 2.4 | 1.9 | 2.0 | 2.0 | 1.0 |
| No | 93.0 | 93.8 | 94,9 | 95.5 | 96.3 | 97.5 |
| Headache | ||||||
| Yes | 1.0 | 1.3 | 0.9 | 1.4 | 1.4 | 1.8 |
| No | 95.0 | 94.8 | 95.9 | 96.2 | 96.8 | 97.4 |
| Bone pain | ||||||
| Yes | 0.7 | 0.9 | 0.5 | 0.4 | 0.4 | 0.8 |
| No | 95.2 | 95.3 | 96.3 | 97.2 | 97.9 | 98.3 |
Values are percentage of the donor reports.
Malignancies during the follow-up period after PBSC donation
| Interval after PBPC donation/localization . | Sex/age, y . | Incidence in Germany (per 100 000/y) . | Crude rate . | SIR . | 95% CI . |
|---|---|---|---|---|---|
| 6 mo | |||||
| Malignant melanoma | Male/49 | 13.95 | 12.14 | 1.14 | 0.03-6.36 |
| 12 mo | |||||
| Neuroendocrine carcinoma of unknown primary localization | Male/33 | 1-1.56 | 12.14 | No data available | |
| 24 mo | |||||
| Adenocarcinoma of rectum | Male/57 | 130.55 | 12.14 | 1.21 | 0.04-6.73 |
| Breast cancer | Female/44 | 105.45 | 39.61 | 0.67 | 0.02-3.75 |
| Hodgkin disease | Male/38 | 2.45 | 24.28 | 8.86 | 1.06-31.98* |
| Hodgkin disease | Male/49 | 2.35 | 24.28 | 8.86 | 1.06-31.98* |
| Testicular carcinoma | Male/39 | 305 | 35.01 | 1.61 | 0.19-5.81 |
| Brain tumor | Male/40 | 4.187 | 12.14 | No data available | |
| AML | Male/35 | 1.08 | 12.14 | 11.13 | 0.33-61.89 |
| 36 mo | |||||
| CLL | Female/43 | 39 | 12.14 | 23.33 | 0.70-129.93 |
| Seminoma | Male/41 | 255 | 35.01 | 1.61 | 0.19-5.81 |
| 48 mo | |||||
| Malignancy of unknown origin | Male/58 | Not applicable |
| Interval after PBPC donation/localization . | Sex/age, y . | Incidence in Germany (per 100 000/y) . | Crude rate . | SIR . | 95% CI . |
|---|---|---|---|---|---|
| 6 mo | |||||
| Malignant melanoma | Male/49 | 13.95 | 12.14 | 1.14 | 0.03-6.36 |
| 12 mo | |||||
| Neuroendocrine carcinoma of unknown primary localization | Male/33 | 1-1.56 | 12.14 | No data available | |
| 24 mo | |||||
| Adenocarcinoma of rectum | Male/57 | 130.55 | 12.14 | 1.21 | 0.04-6.73 |
| Breast cancer | Female/44 | 105.45 | 39.61 | 0.67 | 0.02-3.75 |
| Hodgkin disease | Male/38 | 2.45 | 24.28 | 8.86 | 1.06-31.98* |
| Hodgkin disease | Male/49 | 2.35 | 24.28 | 8.86 | 1.06-31.98* |
| Testicular carcinoma | Male/39 | 305 | 35.01 | 1.61 | 0.19-5.81 |
| Brain tumor | Male/40 | 4.187 | 12.14 | No data available | |
| AML | Male/35 | 1.08 | 12.14 | 11.13 | 0.33-61.89 |
| 36 mo | |||||
| CLL | Female/43 | 39 | 12.14 | 23.33 | 0.70-129.93 |
| Seminoma | Male/41 | 255 | 35.01 | 1.61 | 0.19-5.81 |
| 48 mo | |||||
| Malignancy of unknown origin | Male/58 | Not applicable |
SIR indicates standardized incidence ratio; and CI, confidence interval.
Discussion
The last decade has witnessed an increasing use of rhG-CSF for PBSC mobilization in healthy allogeneic related and unrelated donors. Thus more data regarding short- and long-term sequels of rhG-CSF administration and leukapheresis have become available.11-13 This study has been the largest prospective series of healthy unrelated donors supervised and followed up by a single apheresis center. The schedules for rhG-CSF administration and leukapheresis were the same in the majority (97.3%) of the donors. With the dose of 7.5 μg/kg lenograstim and performing large-volume aphereses, a sufficient number of CD34+ cells could be collected in most cases. Only in 0.45% of the donors did “mobilization failure” (CD34+ yield < 2 × 106/kg recipient body weight) occur. This proportion is below the rates reported by other groups.14 The median CD34+ yield from the first apheresis was in the same range as from protocols using daily doses of 10 or 12 μg/kg rhG-CSF.11,13,15 The frequency with which a second PBSC collection was needed (21.8%) in our unrelated donor population was very similar to the results we have seen in related donors, of whom 25.5% (112 of 328) needed a second apheresis during the same observation period (K.H., M.K., M. Blechschmidt, et al, manuscript in preparation). In accordance with the literature, mobilization and harvest of CD34+ cells were significantly more effective in male than in female donors.16-18 Future research could identify genetic factors that would predict which donors are likely to fail adequate mobilization. One could envision alternative mobilization regimens19 or decide for bone marrow collection.
The side effects of rhG-CSF administration and leukapheresis were consistent with those previously described by other centers.11,13,15,20 It should be emphasized that no donor interrupted the mobilizing protocol and no serious adverse event (including splenic rupture) occurred during mobilization and leukapheresis. This may have been partly because of our very low use of femoral venous access (0.6%). To the best of our knowledge, this is the lowest frequency reported so far and may be attributed to the young age of our unrelated volunteer donors (median, 34 years) and the use of highly experienced staff. In the study of Leitner et al,16 central venous access was needed in 2 of 171 (1.1%) related donors. This resembles our own experience in 328 related donors with a median age of 49 years (range, 5-74 years), 4 (1.2%) of whom required a central line. Other groups have reported the need for central venous catheters in up to 20% of donations.1,13
Four weeks after PBSC collection, the WBC, ANC, and lymphocyte counts were significantly diminished. Later follow-up estimations (up to 5 years) of WBC and ANC were all within the normal range, although they were consistently slightly below pretreatment values. Although not described in earlier studies,21 this effect has been reported recently by other groups.15,22 From the clinical viewpoint, this very minimal deviation seems not to be significant because it was not associated with a higher rate of infections reported in the follow-up period. It remains speculative whether this long lasting decrease in neutrophils is the result of the depletion of slowly self-renewing hematopoietic stem cells or to a down-modulation of G-CSF receptor or other alterations of the cytokine network. A further explanation for this phenomenon may be a relative increase in WBC and neutrophils at baseline. To become a PBSC donor entails psychologic stress, which could give rise to leukocytosis. To address this possibility, we evaluated a small group of donors who were screened for donation but did not receive rhG-CSF (because transplantation was cancelled for recipient-related causes). In this group of 24 donors, there was also a trend toward decreased WBC, ANC, and platelet counts relative to baseline values, but only the decrease in ANC was statistically significant (data not shown).
Like other authors,15,23,24 we observed decreased lymphocyte counts after PBSC donation. Lymphocytes normalized more quickly after a single leukapheresis and in the whole donor population, complete normalization after 2 years was observed. Because of their slower turnover, lymphocytes could conceivably be depleted in the long-term by large-volume leukapheresis (with or without rhG-CSF administration). This possibility should be considered when developing guidelines for stem cell collection. Among the donors under consideration, no complications relatable to lymphopenia, such as the development of infection, have been observed. Between 3 and 5 years after PBSC donation, lymphocyte counts were actually slightly but significantly elevated compared with baseline values. This phenomenon has not been reported earlier and would need to be confirmed in further studies before conclusions could be drawn.
Administration of rhG-CSF and leukapheresis both substantially reduce platelet counts. To avoid any risk for the donor, we retransfused autologous platelets separated from the first PBSC harvest if the platelet count after apheresis had fallen to less than 50 × 109/L. No significant bleeding episode occurred. Nevertheless, the use of acetylsalicylic acid (aspirin) should be avoided for approximately 10 days preceding and after PBSC collection. A slight reduction in platelet counts was detected 4 weeks after PBSC harvest and normalized during further follow-up. This might be attributable to a temporary suppression of megakaryopoiesis by rhG-CSF, but also to the platelet depletion by the leukapheresis procedure itself.13,25 Besides that, we and others have shown that the spleen becomes significantly enlarged by rhG-CSF administration26,27 and conceivably changes in spleen size and function may affect platelet counts.
During the follow-up, 12 donors (0.3%) developed malignant diseases, 4 of which were hematologic malignancies (2 Hodgkin disease, 1 CLL, and 1 AML). Referring to the standardized incidence ratio and the respective confidence interval, the incidence of Hodgkin disease in the cohort of donors investigated turned out to be significantly different from the natural incidence in the German population. For AML and CLL, as for other malignancies, there was no significant difference compared with age-adjusted populations.5,7-9 Although some groups did not observe hematologic malignancies in healthy PBSC donors,15,28-30 there have been reports of AML in related donors.31,32 The National Marrow Donor Program of the United States recently reported follow-up data of 4015 donors who completed more than 1 year after their PBSC donation (9785 donor-years) with no cases of leukemia or lymphoma.33 Data on hematologic malignancies in donors of the German Bone Marrow Donor Center have been reported earlier (G. Ehninger and A. Schmidt, written communication, presented at the EBMT Meeting, 2007). Halter et al recently published a retrospective multicenter EBMT study covering 51 024 first allogeneic hematopoietic stem cell donations (27 770 bone marrow and 23 254 peripheral blood).34 In this survey, 20 hematologic malignancies were reported (8 in bone marrow donors, 12 in PBSC donors). The incidences in both groups were below the age-specific incidence of the normal population, but the authors suspect underreporting because of the retrospective and heterogeneous character of the data. Only one case of hematologic neoplasia (AML) was reported in an unrelated bone marrow donor, whereas 15 cases were in related donors, which represented the majority in that study.
One major question, whether rhG-CSF administration contributes to leukemogenesis, cannot be answered at the moment. In vitro data35-38 about epigenetic and genetic alterations in lymphocytes, changes in gene expression patterns in mononuclear cells, and DNA destabilization have raised more concern regarding this issue. Further studies investigating chromosomal alterations in healthy PBSC donors are needed and underway12 (C. Thiede, Dresden University, oral communication, March 6, 2008). Regarding the incidence of Hodgkin disease after PBSC mobilization, there are only anecdotal reports in the literature. The survey of Halter et al included 1 case of Hodgkin disease; another case has been reported by Leitner et al, both in male PBSC donors who were siblings of patients with hematologic malignancies.16,34 The sample size investigated in our study is still far too small to allow any conclusion to be drawn. Therefore, although our data provide an interim analysis, they call for a prolonged and thorough follow-up of the increasing number of volunteer donors receiving rhG-CSF for PBSC mobilization.
In conclusion, we have determined, from monitoring a large group of healthy unrelated donors, that hematopoietic stem cells can be mobilized into the bloodstream with a moderate dose of rhG-CSF, and collected with a clinically adequate CD34+ yield and without short-term serious side effects. Persistent changes in WBC may occur but to date have not been found to be clinically significant. Long-term monitoring of donor safety is important to establish guidelines as to how often rhG-CSF should be administered and leukapheresis performed. Other forms of leukocyte donation, such as lymphocytapheresis and granulocyte collection, should be included in these monitoring programs as well. The excellent acceptance by our PBSC donors encourages us to continue our efforts to evaluate these procedures.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the altruistic generosity of the unrelated allogeneic PBSC volunteer donors; the nurses and physicians of the apheresis units at the Department of Transfusion Medicine and Cellex GmbH for very skillful medical care to the donors, without whose ongoing commitment this work would not have been possible; and M. A. Meredyth-Stewart for critically reading the manuscript.
Authorship
Contribution: K.H. and G.E. designed the current analysis; M.K. and T.M. collected and analyzed the data; K.H. and M.K. made the figures; and K.H., M.B., and M.K. wrote the paper. All authors contributed to data analysis and interpretation and to the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kristina Hölig, Department of Internal Medicine I, University Hospital Carl Gustav Carus, TU-Dresden, Fetscherstrasse 74, D-01307, Dresden, Germany; e-mail: kristina.hoelig@uniklinikum-dresden.de.