Abstract
Hypereosinophilic syndromes (HESs) are a heterogeneous group of uncommon disorders characterized by marked peripheral eosinophilia and end organ manifestations attributable to the eosinophilia or unexplained in the clinical setting. Whereas corticosteroids remain the mainstay of treatment for most patients, recent diagnostic advances and the development of novel targeted therapies, including tyrosine kinase inhibitors and humanized monoclonal antibodies, have increased the complexity of therapeutic decisions in HESs. This review presents a treatment-based approach to the diagnosis and classification of patients with peripheral blood eosinophilia of 1.5 × 109/L (1500/mm3) or higher and discusses the role of currently available therapeutic agents in the treatment of these patients.
Introduction
The idiopathic hypereosinophilic syndrome (HES) was first defined by Chusid et al in 1975 as (1) persistent eosinophilia of 1.5 × 109/L or more eosinophils (≥1500 eosinophils/mm3) for longer than 6 months, or death before 6 months associated with signs and symptoms of hypereosinophilic disease; (2) a lack of evidence for parasites, allergies, or other known causes of eosinophilia; and (3) presumptive signs and symptoms of organ involvement.1 Although it was recognized at the time that this definition included a spectrum of disorders that varied considerably in their clinical manifestations, responses to treatment, and prognosis, few diagnostic tests were available to reliably distinguish among potential HES variants. Consequently, therapeutic decisions were limited. Corticosteroids were first-line therapy for most patients meeting criteria for HES. Alternative therapies for patients failing corticosteroid therapy included cytotoxic agents, such as hydroxyurea and vincristine, and immunomodulatory agents, of which interferon-α showed the most promise.2
Despite aggressive therapy, some patients with HES developed severe, often fatal, complications, including endomyocardial fibrosis and neurologic involvement. Many of these patients were men with markedly elevated leukocyte counts, anemia, thrombocytopenia, splenomegaly, and other features of myeloproliferative disease. The discovery of the fusion tyrosine kinase FIP1L1/PDGFRA (F/P),3 the most common mutation associated with this “myeloproliferative variant” HES (M-HES),4 both confirmed that a subset of HES patients has a form of chronic eosinophilic leukemia (CEL) and provided an explanation for the response of these patients to the tyrosine kinase inhibitor, imatinib. This has dramatically altered the approach to treatment in HES. Similarly, the identification of a second distinct subset of HES patients with lymphocytic variant HES (L-HES) in whom eosinophilia is due to the secretion of eosinophilopoietic cytokines by phenotypically aberrant populations of T cells (as defined by flow cytometry)5,6 has had important implications with respect to treatment choice7 and monitoring8,9 for this subgroup of HES patients.
As additional HES variants are identified and the number of targeted therapies continues to expand, it will becoming increasingly important to identify the HES variants most likely to respond to specific therapies and to define the long-term efficacy and toxicities of these agents. This review presents an approach to the diagnosis and treatment of patients presenting with eosinophilia of 1.5 × 109/L or higher that is based on the current state of knowledge with respect to the etiologies of HES and available therapies.
Confirmation of the diagnosis of HES
Because the differential diagnosis of eosinophilia of 1.5 × 109/L or higher is quite broad, the most important step in treating HES is excluding disorders associated with secondary eosinophilia that require specific therapies not directed primarily at the eosinophilia. These include parasitic infections, drug hypersensitivity reactions, and neoplasms (Table 1). Although the optimal evaluation will differ for individual patients and is beyond the scope of this review, several general principles warrant mention.
Causes of marked eosinophilia other than HES
| Category . | Examples (noninclusive) . |
|---|---|
| Allergic disorders* | Asthma, atopic dermatitis |
| Drug hypersensitivity | Varied |
| Infection | |
| Helminth | Varied, including strongyloidiasis, hookworm infection, filariasis |
| Ectoparasite | Scabies, myiasis |
| Protozoan | Isosporiasis, Sarcocystis myositis |
| Bacterial | Chronic tuberculosis, resolving scarlet fever |
| Fungal | Varied, including coccidiomycosis, allergic bronchopulmonary aspergillosis |
| Viral | HIV |
| Neoplasm | Leukemia, lymphoma, solid organ adenocarcinoma |
| Autoimmune and idiopathic disorders† | Connective tissue disorders, sarcoidosis, inflammatory bowel disease, autoimmune lymphoproliferative disorder |
| Other | Hypoadrenalism, radiation exposure, cholesterol embolization, IL-2 therapy |
| Category . | Examples (noninclusive) . |
|---|---|
| Allergic disorders* | Asthma, atopic dermatitis |
| Drug hypersensitivity | Varied |
| Infection | |
| Helminth | Varied, including strongyloidiasis, hookworm infection, filariasis |
| Ectoparasite | Scabies, myiasis |
| Protozoan | Isosporiasis, Sarcocystis myositis |
| Bacterial | Chronic tuberculosis, resolving scarlet fever |
| Fungal | Varied, including coccidiomycosis, allergic bronchopulmonary aspergillosis |
| Viral | HIV |
| Neoplasm | Leukemia, lymphoma, solid organ adenocarcinoma |
| Autoimmune and idiopathic disorders† | Connective tissue disorders, sarcoidosis, inflammatory bowel disease, autoimmune lymphoproliferative disorder |
| Other | Hypoadrenalism, radiation exposure, cholesterol embolization, IL-2 therapy |
HES indicates hypereosinophilic syndrome.
Allergic disorders, including asthma and atopic dermatitis, are common in patients with lymphocytic variant HES (L-HES) and idiopathic HES. Consequently, the distinction between allergic disease with marked eosinophilia and HES with concomitant allergic disease may be impossible.
Marked peripheral blood eosinophilia can occur in the setting of a wide variety of autoimmune and idiopathic disorders, particularly those characterized by abnormal lymphocyte proliferation or activation. Signs and symptoms of HES are infrequent and can be difficult to distinguish from manifestations of the underlying disorder.
First, the clinical manifestations of HES can be indistinguishable from those due to marked eosinophilia of other causes. Endomyocardial fibrosis, for example, has been reported in association with eosinophilia in a wide variety of disorders, including Loa loa infection10 and adenocarcinoma of the lung.11 Second, drug hypersensitivity reactions should always be considered early in the evaluation of unexplained eosinophilia. Although some agents are associated with specific clinical syndromes, such as semisynthetic penicillins and interstitial nephritis, the clinical manifestations of drug-induced eosinophilia are often indistinguishable from those of HES. Furthermore, the list of agents that have been associated with eosinophilia is extensive and includes prescription and nonprescription drugs as well as dietary supplements and herbal remedies. Consequently, all nonessential agents should be discontinued before a diagnosis of HES is made. Third, evaluation for a parasitic cause of eosinophilia should be dictated by the exposure history, clinical signs, and symptoms. The exception is strongyloidiasis, which is often asymptomatic and is endemic worldwide. Because of the possibility that patients treated with steroids can develop hyperinfection syndrome, a potentially fatal form of strongyloidiasis, Strongyloides infection should be excluded by serologic testing in all patients with eosinophilia and a plausible history of exposure. Finally, eosinophilia can precede other clinical manifestations of an occult neoplasm, sometimes by many years. Thus, patients suspected of having HES should be evaluated with radiologic studies, bone marrow examination, and other testing for neoplasia as appropriate depending on the patient's age, sex, and other risk factors.
Because the identification of the F/P chromosomal mutation in patients with M-HES and the demonstration of clonal T cells in patients with L-HES, there has been controversy over the classification of hypereosinophilic syndromes and whether these clinical entities, reclassified in the 2008 World Health Organization (WHO) guidelines as myeloid neoplasms and peripheral T-cell lymphoma not otherwise specified, respectively,12,13 should be considered part of the continuum of HES. Although strictly speaking, the new WHO classification is correct, I believe that there is value in grouping patients by clinical presentation with respect to treatment decisions. This is a similar approach to that of Chusid et al and his contemporaries, who viewed HES as “a continuum of hypereosinophilic disease with eosinophilic leukemia existing at one pole.”1(p1)
Differentiation of HES subtypes
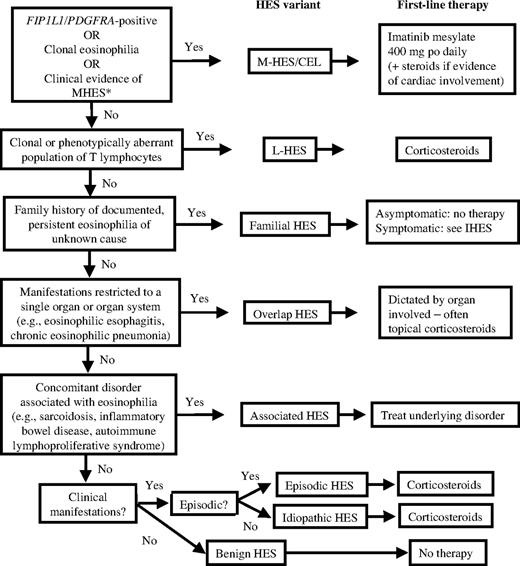
At a 2005 international consensus workshop on the treatment of HES, a classification scheme was proposed with the goal of subdividing patients meeting the definition of HES into clinical groups (or variants) to facilitate the approach to treatment.14 Six clinical groups were described: (1) myeloproliferative variant HES (including F/P-negative HES with myeloproliferative features, F/P-positive HES/CEL, and CEL with cytogenetic abnormalities and/or increased blasts); (2) lymphocytic variant HES (HES with a demonstrable clonal or phenotypically aberrant lymphocyte population); (3) familial eosinophilia (including an autosomal dominant form of marked eosinophilia that has been mapped to chromosome 5q31-3315 ); (4) undefined HES (idiopathic HES with or without symptoms, including episodic variants); (5) overlap HES (eosinophilic disease restricted to a single organ system accompanied by peripheral eosinophilia ≥ 1.5 × 109/L) and (6) associated HES (eosinophilia ≥ 1.5 × 109/L in the setting of another diagnosis, such as sarcoidosis or inflammatory bowel disease, in which eosinophilia has been described as a feature in a subset of affected patients). Although the proposed scheme is far from perfect and is likely to evolve as additional diagnostic tools become available, it provides a framework upon which to base treatment decisions.
General approach to treatment
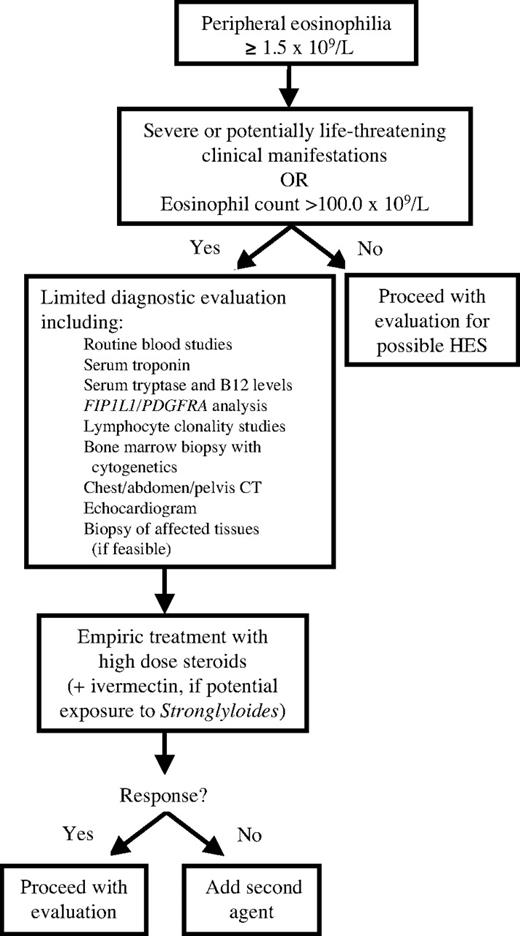
The first question to address with respect to treatment of HES is whether the patient requires urgent intervention (Figure 1). Patients presenting with potentially life-threatening complications, including cardiac or neurologic involvement, and marked eosinophilia should be treated empirically with high-dose corticosteroids (eg, intravenous methylprednisolone at a dose of 1 mg/kg per day) to prevent progression of end organ damage. Although every effort should be made to obtain necessary diagnostic studies, including blood work, imaging studies, and biopsies of affected tissues before initiating corticosteroid therapy, treatment should not be delayed in the face of worsening signs and symptoms. Empiric double-dose ivermectin (200 μg/kg × 2 days) should be given to patients with potential exposure to Strongyloides.
In patients with aggressive disease unresponsive to several days of high-dose corticosteroids, addition of a second agent should be guided by the clinical presentation. Imatinib therapy should be considered early in a male patient presenting with new onset myocarditis and marked eosinophilia; whereas a female patient with a history of asthma and nasal polyps presenting with myocarditis and dramatic eosinophilia would be more likely to have Churg-Strauss vasculitis and to benefit from sustained corticosteroid therapy. Vincristine, used to rapidly lower eosinophil counts in patients with HES, should be reserved for patients with rapidly progressive, life-threatening disease unresponsive to high-dose steroids and imatinib therapy.
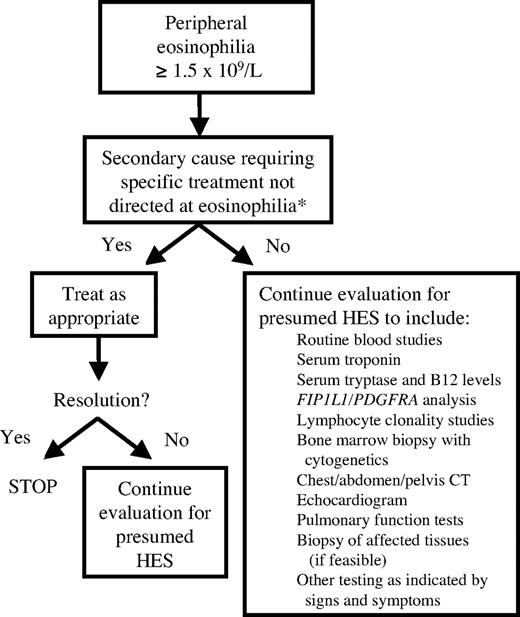
Once the patient is clinically stable, the evaluation should proceed with the goal of confirming the diagnosis of HES and classifying the patient into one of the described HES variants (Figure 2). Treatment of M-HES, L-HES, and idiopathic HES will be discussed in “Treatment of HES variants.” Distinguishing between these disorders and the associated or overlap syndromes can be extremely difficult and is beyond the scope of this review. At the present time, there are no data to suggest that familial eosinophilia requires treatment unless signs and symptoms of organ involvement become apparent,15 in which case the approach would be similar to that for treatment of undefined HES.
Evaluation of the patient with HES. *Secondary causes include, but are not limited to, drug hypersensitivity, infection, hypoadrenalism, and solid organ neoplasm.
Evaluation of the patient with HES. *Secondary causes include, but are not limited to, drug hypersensitivity, infection, hypoadrenalism, and solid organ neoplasm.
Regardless of the etiology of the eosinophilia, patients should be monitored at least at 6-month intervals for progression of organ involvement. In addition to the evaluation of known affected organ systems, occult cardiac and/or pulmonary involvement should be assessed with serum troponin levels, electrocardiogram, echocardiography, and pulmonary function testing.
Treatment of HES variants
Myeloproliferative HES/CEL
The availability of the tyrosine kinase inhibitor, imatinib mesylate, has led to a dramatic decrease in the morbidity and mortality of F/P-positive HES/CEL (Figure 3). Response rates approach 100% in most series, and primary drug resistance is rare.16 Consequently, imatinib has become the first-line treatment of choice for this condition, irrespective of the severity of clinical manifestations. Although the utility of imatinib therapy in patients who do not have a detectable PDGFRA fusion gene remains controversial,17 a trial of imatinib should be considered in patients presenting with aggressive disease unresponsive to corticosteroids and/or features of myeloproliferative disease. Of note, D816KIT-positive systemic mastocytosis overlaps in clinical presentation with F/P-positive HES/CEL but is resistant to imatinib.18,19 The 2 disorders can most reliably be distinguished by molecular testing, although a clinical scoring index has been developed that can be useful if such testing is not available.20
Treatment-based approach to the patient with presumed HES. *Four or more of the following: dysplastic eosinophils, serum B12 > 737.8 pM (1000 pg/mL), serum tryptase > 12 ng/mL, anemia and/or thrombocytopenia, splenomegaly, bone marrow cellularity > 80%, myelofibrosis, spindle-shaped mast cells > 25%.
Treatment-based approach to the patient with presumed HES. *Four or more of the following: dysplastic eosinophils, serum B12 > 737.8 pM (1000 pg/mL), serum tryptase > 12 ng/mL, anemia and/or thrombocytopenia, splenomegaly, bone marrow cellularity > 80%, myelofibrosis, spindle-shaped mast cells > 25%.
The F/P fusion is 50-fold more sensitive to imatinib than bcr/abl,3 and molecular remission can be maintained in most patients with F/P-positive disease with low doses of imatinib (as little as 100 mg/week in some series21 ). Nevertheless, data from chronic myelogenous leukemia (CML) suggest that initiation of therapy with higher doses (eg, 400 mg/day) leads to longer remission. Thus, it seems prudent to initiate therapy with imatinib 400 mg/day and to taper the dose only after a complete and stable clinical, hematologic, and molecular remission has been achieved.
Rapid institution of therapy is important to prevent irreversible damage, including endomyocardial fibrosis and sequelae of thromboembolic events.22 The side-effect profile of imatinib in HES/CEL appears to be similar to that described for CML with the exception of acute necrotizing myocarditis, a rare complication described in patients with HES and preexistent cardiac involvement.23,24 Consequently, it is recommended that patients with known cardiac manifestations or an elevated serum troponin24 receive high-dose corticosteroids during the first 2 weeks of imatinib therapy.
In patients with PDGFRA-associated disease, the response to imatinib therapy is extremely rapid with resolution of eosinophilia and improvement in clinical symptoms occurring within the first week of therapy and normalization of bone marrow studies within the first month. Molecular remission occurs in most, if not all, patients, but may take considerably longer (12-18 months in some cases).25,26 Although the kinetics of response tends to be slower in patients without the F/P mutation and higher doses of imatinib may be necessary,27 persistent eosinophilia of 1.5 × 109/L or higher after 1 month of treatment should be considered a treatment failure. Repeat bone marrow examination should be performed in all patients within the first several months of imatinib therapy to confirm remission. This is particularly true for F/P mutation–negative patients experiencing a suboptimal or partial response to therapy, as unmasking of pre-B-cell acute lymphoblastic leukemia by imatinib has been reported in at least 1 patient presenting with characteristic clinical manifestations of HES.28
Once a stable dosing regimen is achieved, efficacy should be monitored with monthly eosinophil counts and regular assessment of organ involvement. Because molecular relapse typically occurs before the recurrence of eosinophilia and clinical manifestations,29 F/P-positive patients should be tested for the presence of the fusion gene every 3 to 6 months. A positive test should raise concern for drug resistance and prompt an increase in imatinib dose.
Imatinib therapy controls, but does not cure, HES/CEL,29 and isolated instances of drug resistance have been reported.3,26 Other tyrosine kinase inhibitors effective in the treatment of CML, including nilotinib30 and sorafenib,31 have been demonstrated to have activity against F/P in vitro and are likely to be useful clinically in the event of imatinib resistance. Bone marrow transplantation remains the only curative option and has been used successfully in F/P-positive HES/CEL,32 but should be reserved for patients with aggressive disease unresponsive to tyrosine kinase inhibition.
Lymphocytic variant HES
Corticosteroids are the first-line therapy for symptomatic L-HES, and most patients respond rapidly to moderate- to high-dose therapy (30-60 mg prednisone equivalent daily). Once the eosinophil count has normalized and symptoms have improved, slow tapering to 10 mg or less prednisone equivalent daily should be attempted. In patients who experience significant corticosteroid side effects or who fail to respond adequately to therapy, a second agent should be added. Among commercially available steroid-sparing agents, interferon-α has effects on both eosinophils and T cells and has been used most extensively. Because of theoretic considerations that interferon-α monotherapy could lead to outgrowth of the abnormal lymphocyte populations in some patients,7,10 it has been suggested that interferon-α be administered only in combination with glucocorticoids in patients with proven L-HES.
Alternative agents for which there is some evidence of efficacy in L-HES include mepolizumab33 and alemtuzumab.34 In contrast, data to date suggest that imatinib is not effective in L-HES, although it should be noted that rare cases of T-cell clones accompanying F/P-positive HES/CEL have been reported.35
Because of the increased risk of development of T-cell lymphoma in patients with L-HES, lymphocyte counts should be followed every 3 months and the proportion of aberrant T cells assessed by peripheral blood flow cytometry every 6 months. Follow-up bone marrow examination with cytogenetics should be performed at regular intervals (every 1-2 years) as cytogenetic abnormalities, particularly 6q− chromosomal deletions, may be an early marker of progression to lymphoma.8
Undefined HES
Despite comprehensive evaluation, more than 50% of patients with HES cannot be classified into either of the 2 previously discussed categories. Such patients can, however, be divided into 3 groups on the basis of clinical manifestations: benign (without evidence of end organ involvement, including subclinical cardiac involvement as assessed by serum troponin levels), complex (with evidence of multisystem involvement), and episodic (with clinical signs and symptoms that remit and recur spontaneously).
Although patients with prolonged asymptomatic eosinophilia of 1.5 × 109/L or higher (benign eosinophilia) certainly exist, it may be difficult at the time of presentation to distinguish these patients from patients with early HES who will go on to develop clinical manifestations. A variety of clinical markers of disease progression have been proposed, including serum levels of eosinophil granule proteins; however, none has been validated to date. Thus, because the agents used to treat HES are not without toxicity and can be very costly, it seems prudent to follow clinically asymptomatic patients with idiopathic HES closely without treatment for the development of signs and symptoms.
The mainstay of treatment for symptomatic idiopathic HES, whether constant or episodic, remains glucocorticoids. Whereas the majority of patients will respond, at least initially, to high-dose corticosteroids (eg, prednisone 1 mg/kg daily), the most appropriate starting and maintenance doses are unknown and should be guided by the clinical manifestations in the individual patient. For example, a patient with aggressive disease or in whom Churg-Strauss vasculitis is suspected should be treated with high-dose corticosteroids for a minimum of 2 to 4 weeks before a slow taper to the lowest dose at which symptoms and eosinophilia remain suppressed. Conversely, a patient with dermatologic manifestations and no other organ involvement could be started on low-dose therapy (10-20 mg prednisone) with dose adjustment depending on the clinical response. In patients with gastrointestinal symptoms who do not respond to oral corticosteroids, a short course of intravenous therapy should be considered to ensure absorption with conversion to oral steroids once a clinical response is achieved.
As in L-HES, a slow taper to 10 mg or less prednisone equivalent should be attempted. Patients requiring higher corticosteroid doses, or in whom significant toxicity develops, should receive a second agent. Daily hydroxyurea (1-2 g orally) and interferon-α (1-3 mU subcutaneously), the most widely used agents, are each effective in approximately 30% of patients, although toxicity is common.36 Low-dose hydroxyurea (500 mg daily) appears to potentiate the effects of interferon-α without increasing its toxicity37 and is a reasonable alternative. A trial of imatinib should be considered for steroid-refractory patients, as rare responses in F/P-negative patients have been reported.17,27 Several additional agents, including chlorambucil, vincristine, etoposide, cladribine, cytarabine, methotrexate, cyclosporine, and cyclophosphamide, have been used to treat small numbers of steroid-unresponsive patients with varied success, but cannot be routinely recommended.
Among the agents in clinical development, mepolizumab (GlaxoSmithKline), a humanized monoclonal anti–IL-5 antibody, has been the best studied. After promising results in pilot studies, a double-blind, placebo-controlled trial in 85 F/P mutation–negative patients demonstrated that monthly mepolizumab was safe and effective as a steroid-sparing agent in HES.38 Mepolizumab is currently available only for compassionate use in patients with life-threatening HES refractory to standard therapies (http://www.clinicaltrials.gov).
Conclusions
HESs are a heterogeneous group of uncommon disorders, ranging from benign idiopathic eosinophilia to eosinophilic leukemia. Consequently, therapeutic choices should be guided not only by the severity of the clinical manifestations and the side-effect profile of the agents, but by the underlying etiology of the eosinophilia. As the number of new and expensive targeted therapies continues to grow, a logical etiology-based approach to treatment will become increasingly important.
Acknowledgments
The author thanks Drs Peter Weller, Thomas Nutman, and Princess Ogbogu for their critical review of the paper.
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID).
National Institutes of Health
Authorship
Contribution: A.D.K. was the sole author of this paper.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Amy D. Klion, Laboratory of Parasitic Diseases, National Institutes of Health, 9000 Rockville Pike, Bldg 50, Rm 6351, Bethesda, MD 20892; e-mail: aklion@niaid.nih.gov.