Abstract
Hyperactive RAS signaling is caused by mutations in RAS genes or a deficiency of the neurofibromatosis gene (NF1) and is common in myeloid malignancies. In mice, expression of oncogenic K-RAS or inactivation of Nf1 in hematopoietic cells results in myeloproliferative disorders (MPDs) that do not progress to acute myeloid leukemia (AML). Because NF1 is a RAS-GTPase–activating protein it has been proposed that NF1 deficiency is functionally equivalent to an oncogenic RAS. It is not clear, however, whether Nf1 deficiency would be redundant in K-RAS–induced MPD development or whether the 2 mutations would cooperate in leukemogenesis. Here, we show that the simultaneous inactivation of Nf1 and expression of K-RASG12D in mouse hematopoietic cells results in AML that was fatal in primary mice within 4 weeks and transplantable to sublethally irradiated secondary recipients. The data point to a strong cooperation between Nf1 deficiency and oncogenic K-RAS.
Introduction
Hyperactive RAS signaling is associated with the development of myeloid malignancies and can be caused by a deficiency in the neurofibromatosis type 1 gene (NF1) or by oncogenic mutations in RAS genes. Children with NF1 deficiency have an increased risk of developing juvenile myelomonocytic leukemia (JMML) and oncogenic mutations in RAS proteins are common in JMML and chronic myelomonocytic leukemia.1 JMML and chronic myelomonocytic leukemia are aggressive myeloproliferative disorders (MPDs) that can progress to acute myeloid leukemia (AML) but usually only together with other genetic alterations. Thus, hyperactive signaling through the RAS proteins is likely an initiating event in the development of MPD.
This reasoning draws support from mouse models of hyperactive RAS signaling: Conditional inactivation of Nf1 in hematopoietic cells produces a fatal MPD with a long latency2 ; similarly, conditional expression of oncogenic K-RAS in hematopoietic cells produces a fatal MPD, but with a shorter latency.3,4 Both models exhibit growth factor hypersensitivity of hematopoietic cells, splenomegaly, and infiltration of myeloid cells in the liver—but they do not progress to acute leukemia, unless accompanied by other genetic alterations.5,6
NF1 encodes neurofibromin, a GTPase-activating protein for the RAS proteins.7 Thus, NF1-deficient cells have increased levels of RAS-GTP that result in hyperactive RAS signaling. Consequently, NF1 deficiency has been viewed as functionally equivalent to an oncogenic RAS mutation. This is supported by the fact that NF1 and RAS mutations are not found in the same patient.8 But NF1 may also be involved in other signaling pathways.9-11 At this point, the impact of Nf1 deficiency in the setting of an oncogenic RAS in bone marrow cells is unknown.
The MPD induced by Nf1 deficiency in mice is less severe than the K-RAS–induced MPD. We argued that if Nf1 deficiency was functionally equivalent to an oncogenic RAS, then inactivating Nf1 should have a limited effect on the development of K-RAS–induced MPD. To approach this issue, we bred Nf1 conditional knockout mice on a background of the latent oncogenic K-RAS allele and the Mx1-Cre transgene. After Cre recombination in the triple-transgenic mice, we could define the impact of simultaneously inactivating Nf1 and expressing K-RASG12D in hematopoietic cells.
Methods
Mouse breeding and in vivo experiments
Mice with conditional Nf1fl alleles12 (designated N) were bred with Kras2LSL mice13 (designated K) to generate NK mice. NK mice were bred with mice harboring the interferon-inducible Mx1-Cre transgene14 (designated M) to generate NKM mice. NKM mice were compared with littermate NM and KM mice (ie, MPD controls); K and N mice were used as healthy controls (designated Ctr).
Groups of 4-week-old mice were injected with 200 μg polyinosinic-polycytidylic acid (pI-pC; Sigma). Blood was analyzed with a Hemavet 950FS (Drew Scientific) and by manual differential counts. For transplantations, bone marrow (BM) cells were harvested 3 weeks after pI-pC treatment and injected into sublethally irradiated (650 cGy) wild-type mice. Animal procedures were approved by the Animal Research Ethics Committee in Gothenburg, Sweden.
Fluorescence-activated cell sorting, colony assays, histology, and Western blots
Splenocytes and BM cells were incubated with antibodies and analyzed in a FACSAria (BD Biosciences). Colony assays, histology, isolation of CD11b+ cells, and Western blots were performed as described.15 RAS-GTP was analyzed with the Active RAS Pull-Down and Detection kit (89955; Thermo Scientific).
Quantitative PCR
cDNA was synthesized from total RNA from CD11b+ cells with the iScript cDNA Kit (Bio-Rad) and was used for real-time polymerase chain reaction (PCR) with the Mouse p53 Signaling Pathway PCR Array (PAMM-027; SuperArray Bioscience Corporation), and with primers for Bcl11a, Bcl11b, and Bcl6 in an ABI Prism 7900HT (Applied Biosystems).
Results and discussion
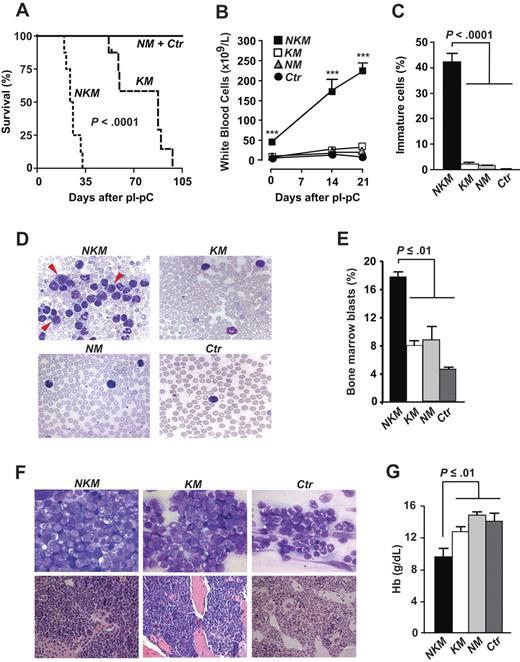
To define the impact of Nf1 deficiency in the setting of K-RASG12D expression in hematopoietic cells, we injected pI-pC into Nf1fl/flKras2LSL/+Mx1-Cre (NKM) mice, MPD control mice (KM and NM), and healthy control mice (Ctr). Consistent with previous studies,3,4,16 all KM mice had died from MPD 98 days after pI-pC injections (median survival, 88 days), whereas NM and Ctr mice lived longer. In contrast, the maximum survival of NKM mice was 33 days (median, 25; P < .001 versus KM; Figure 1A). Twenty-one days after pI-pC injections, white blood cell counts were 233 plus or minus 16 × 109/L in NKM mice compared with mean counts of 34 × 109/L, 21 × 109/L, and 13 × 109/L for KM, NM, and Ctr, respectively (Figure 1B). The NKM mice exhibited high levels of immature cells including myeloblasts (Figure 1C-D) in blood, increased levels of BM blasts (Figure 1E-F), and anemia (Figure 1G). These results suggest that NKM mice develop a myeloid malignancy with a reduced latency and increased severity compared with KM and NM mice.
Survival and analyses of white blood cells and hemoglobin levels of NKM and control mice. (A) Kaplan-Meier survival plots for NKM (n = 8), KM (n = 8), NM (n = 6), and Ctr mice (n = 6). (B) White blood cell counts of NKM, KM, NM, and Ctr mice (n > 8 in each group). ***P < .001. (C) Percentage of immature white blood cells in peripheral blood of NKM (n = 4), KM (n = 3), NM (n = 3), and Ctr (n = 3) mice 3 weeks after pI-pC injections. (D) Photographs of typical blood smears evaluated in panel C (original magnification, ×100; 1.30 NA oil objective). Arrowheads indicate myeloblasts. (E) Percentage of blasts was evaluated in BM smears from NKM (n = 3), KM (n = 3), NM (n = 3), and Ctr (n = 2) mice 3 weeks after pI-pC injections. (F, top panels) Photographs of typical bone marrow smears (May-Grünwald-Giemsa staining) evaluated in panel E (original magnification ×100; 1.30 NA oil objective). (Bottom panels) Photographs of sternum sections (hematoxylin and eosin staining; original magnification ×63; 1.40 NA oil objective). (G) Blood hemoglobin concentrations of mice shown in panel B 3 weeks after pI-pC injections. Data are mean and SEM; survival was assessed by the log-rank test; other statistics, 1-way analysis of variance and Tukey posthoc test for multiple comparisons. Images in panels D and F were obtained with an AxioCam camera mounted on an Axioplan 2 microscope and the AxioVision software (release 4.3; Zeiss).
Survival and analyses of white blood cells and hemoglobin levels of NKM and control mice. (A) Kaplan-Meier survival plots for NKM (n = 8), KM (n = 8), NM (n = 6), and Ctr mice (n = 6). (B) White blood cell counts of NKM, KM, NM, and Ctr mice (n > 8 in each group). ***P < .001. (C) Percentage of immature white blood cells in peripheral blood of NKM (n = 4), KM (n = 3), NM (n = 3), and Ctr (n = 3) mice 3 weeks after pI-pC injections. (D) Photographs of typical blood smears evaluated in panel C (original magnification, ×100; 1.30 NA oil objective). Arrowheads indicate myeloblasts. (E) Percentage of blasts was evaluated in BM smears from NKM (n = 3), KM (n = 3), NM (n = 3), and Ctr (n = 2) mice 3 weeks after pI-pC injections. (F, top panels) Photographs of typical bone marrow smears (May-Grünwald-Giemsa staining) evaluated in panel E (original magnification ×100; 1.30 NA oil objective). (Bottom panels) Photographs of sternum sections (hematoxylin and eosin staining; original magnification ×63; 1.40 NA oil objective). (G) Blood hemoglobin concentrations of mice shown in panel B 3 weeks after pI-pC injections. Data are mean and SEM; survival was assessed by the log-rank test; other statistics, 1-way analysis of variance and Tukey posthoc test for multiple comparisons. Images in panels D and F were obtained with an AxioCam camera mounted on an Axioplan 2 microscope and the AxioVision software (release 4.3; Zeiss).
Peripheral tissues of NKM mice showed infiltration of myeloid cells, and spleen and liver weights were increased 6- to 10-fold and 1.5- to 1.7-fold, respectively, compared with KM, NM, and Ctr mice (Figure 2A-B). Fluorescence-activated cell sorting analyses showed an increased proportion of CD11b+/Gr1+, CD34+, and CD117+ cells in the spleen of NKM mice (Figure 2C). Consequently, splenocytes and BM cells from NKM mice produced more colonies in methylcellulose both in the absence and presence of growth factors compared with the 3 control groups (Figure 2D, and supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The NKM colonies were larger than KM and NM colonies and occasionally contained blastlike cells, but most often contained atypical myelomonocytic cells. PCR genotyping of DNA from individual colonies showed activation of the Kras2G12D allele in NKM and KM colonies and the presence of the deleted Nf1Δ allele in NKM and NM colonies (Figure 2E). Levels of RAS-GTP in BM cells from NKM mice were indistinguishable from KM (Figure 2F). Similar results were found in isolated CD11b+ cells (supplemental Figure 1). Thus, the enhanced disease severity of NKM compared with KM mice cannot be explained by differences in RAS-GTP levels.
NKM mice exhibit massive hepatosplenomegaly, extramedullary hematopoiesis, and increased autonomous and granulocyte-macrophage colony-stimulating factor–induced colony growth of splenocytes. (A) Spleen and (B) liver weight (relative to total body weight [bwt]) in NKM (n = 9), KM (n = 7), NM (n = 4), and Ctr (n = 7) 3 weeks after pI-pC injections. (C) Representative flow cytometry plots of splenocytes with antibodies recognizing CD34, CD117, CD11b, and Gr-1. Mean percentage of double-positive splenocytes from NKM (n = 6), KM (n = 3), NM (n = 1) and Ctr (n = 3) mice is indicated. (D) Granulocyte-macrophage colony-forming unit colony-forming ability of splenocytes isolated from NKM (n = 3), KM (n = 3), NM (n = 2) and Ctr (n = 3) mice 3 weeks after pI-pC injections. The number of colonies formed from NKM splenocytes was significantly increased compared with the other genotypes at all granulocyte-macrophage colony-stimulating factor (GM-CSF) concentrations (P < .05). Data are mean and SEM; statistics: 1-way analysis of variance and Tukey posthoc test. (E) PCR amplification of genomic DNA from individual granulocyte-macrophage colony-forming unit colonies. (F) Western blots of extracts from serum-starved and GM-CSF–stimulated BM cells from NKM (n = 2; pooled), KM (n = 1), NM (n = 4), and Ctr mice (n = 5). Actin was used as a loading control. Densitometry of protein bands was determined with the Quantity One software (Version 4.4.0; Bio-Rad).
NKM mice exhibit massive hepatosplenomegaly, extramedullary hematopoiesis, and increased autonomous and granulocyte-macrophage colony-stimulating factor–induced colony growth of splenocytes. (A) Spleen and (B) liver weight (relative to total body weight [bwt]) in NKM (n = 9), KM (n = 7), NM (n = 4), and Ctr (n = 7) 3 weeks after pI-pC injections. (C) Representative flow cytometry plots of splenocytes with antibodies recognizing CD34, CD117, CD11b, and Gr-1. Mean percentage of double-positive splenocytes from NKM (n = 6), KM (n = 3), NM (n = 1) and Ctr (n = 3) mice is indicated. (D) Granulocyte-macrophage colony-forming unit colony-forming ability of splenocytes isolated from NKM (n = 3), KM (n = 3), NM (n = 2) and Ctr (n = 3) mice 3 weeks after pI-pC injections. The number of colonies formed from NKM splenocytes was significantly increased compared with the other genotypes at all granulocyte-macrophage colony-stimulating factor (GM-CSF) concentrations (P < .05). Data are mean and SEM; statistics: 1-way analysis of variance and Tukey posthoc test. (E) PCR amplification of genomic DNA from individual granulocyte-macrophage colony-forming unit colonies. (F) Western blots of extracts from serum-starved and GM-CSF–stimulated BM cells from NKM (n = 2; pooled), KM (n = 1), NM (n = 4), and Ctr mice (n = 5). Actin was used as a loading control. Densitometry of protein bands was determined with the Quantity One software (Version 4.4.0; Bio-Rad).
The disease in KM and NM mice is not transplantable to sublethally irradiated recipients.2-4 To determine transplantability of NKM cells, BM cells from pI-pC–treated NKM mice were injected into sublethally irradiated recipients: All mice (6/6) had died or become moribund by 12 weeks after transplantation and 3 of these exhibited high white blood cell counts (49-157 × 109/L) and splenomegaly. The disease in NKM mice can be classified as AML based on the following criteria17 : more than 20% immature cells in peripheral blood; anemia; increased levels of nonlymphoid hematopoietic cells in the spleen; rapidly fatal to primary animals; and fatal to sublethally irradiated secondary recipients.
CD11b+ hematopoietic cells from NKM mice exhibited reduced expression of proapoptotic (Apaf1, Bid, Foxo3a, Jun, Stat1) and antiproliferative (Btg2, Ep300, Pten, Sesn2) genes, and 2 genes involved in hematopoietic differentiation (Gadd45a, Mcl1; supplemental Table 1). These alterations could contribute to the leukemogenic potential of the NKM cells. In light of a recent study showing that overexpression of Bcl11a cooperates with Nf1 deficiency in leukemogenesis,5 we assessed Bcl11a expression in NKM cells. However, Bcl11a was not increased in NKM cells.
We conclude that simultaneous inactivation of Nf1 and expression of K-RASG12D induced AML in mice. The likeliest interpretation of this result is that NF1 is involved in non-RAS pathways and that these contribute to AML in the setting of K-RASG12D. However, at this point, we cannot rule out the possibility that the AML is caused by hyperactive RAS signaling, per se. For example, Nf1 deficiency might have no impact on K-RASG12D signaling but could cooperate by deregulating N-RAS and H-RAS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tyler Jacks for the Kras2LSL mice, Luis Parada for the Nf1fl/fl mice, and Aziz Hussein for assistance with histopathology.
This study was supported by grants from the European Research Council, Swedish Medical Research Council, the Swedish Cancer Society, the Swedish Children's Cancer Fund, and Västra Götalandsregionen (M.O.B). M.O.B is a fellow of the Swedish Medical Research Council and the European Hematology Association José Carreras Young Investigator Fellowship Program.
Authorship
Contribution: B.A.C. designed and performed research, analyzed data, and wrote the paper; A.-K.M.S. designed and performed research, analyzed data, and wrote the paper; K.M.E.A. performed research, analyzed data, and wrote the paper; A.M.W. performed research; C.K. performed research and analyzed data; B.S. analyzed data; and M.O.B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Bergo, Wallenberg Laboratory, Institute of Medicine, Sahlgrenska University Hospital, S-413 45 Gothenburg, Sweden; e-mail: martin.bergo@wlab.gu.se.
References
Author notes
B.A.C. and A.-K.M.S. contributed equally to this study, and the order in which they are listed should be considered arbitrary.

![Figure 2. NKM mice exhibit massive hepatosplenomegaly, extramedullary hematopoiesis, and increased autonomous and granulocyte-macrophage colony-stimulating factor–induced colony growth of splenocytes. (A) Spleen and (B) liver weight (relative to total body weight [bwt]) in NKM (n = 9), KM (n = 7), NM (n = 4), and Ctr (n = 7) 3 weeks after pI-pC injections. (C) Representative flow cytometry plots of splenocytes with antibodies recognizing CD34, CD117, CD11b, and Gr-1. Mean percentage of double-positive splenocytes from NKM (n = 6), KM (n = 3), NM (n = 1) and Ctr (n = 3) mice is indicated. (D) Granulocyte-macrophage colony-forming unit colony-forming ability of splenocytes isolated from NKM (n = 3), KM (n = 3), NM (n = 2) and Ctr (n = 3) mice 3 weeks after pI-pC injections. The number of colonies formed from NKM splenocytes was significantly increased compared with the other genotypes at all granulocyte-macrophage colony-stimulating factor (GM-CSF) concentrations (P < .05). Data are mean and SEM; statistics: 1-way analysis of variance and Tukey posthoc test. (E) PCR amplification of genomic DNA from individual granulocyte-macrophage colony-forming unit colonies. (F) Western blots of extracts from serum-starved and GM-CSF–stimulated BM cells from NKM (n = 2; pooled), KM (n = 1), NM (n = 4), and Ctr mice (n = 5). Actin was used as a loading control. Densitometry of protein bands was determined with the Quantity One software (Version 4.4.0; Bio-Rad).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/17/10.1182_blood-2009-02-205146/4/m_zh89990943590002.jpeg?Expires=1763521912&Signature=bYkow9JPi5k-wro9gsVJ3HrjVsXbsghgmmiLK3F5Ya6uFyDZGlgC4quXI8vkktWdvXOcHmqKLiZDsP42GbRYbsIvLwiR93w6iswUZvDbM4SmQOfryZOu6mg-JrGeDicmlxfINybxdf5mp4N7oo164weI1JybhixEeJ98IvcOFIzJWLJUuTQkqEaoi9pxxHqSCJ-s5sMJY0Rxzv8LqlibKTQ9ToCJUlPG52N1-0n8ze9EkJ8t8vPWZK7tkSu9QvDD1l9sE0dEg6HjwIIHvMt59V6bAQ1VVCPFV9KOfls6E9Jy3D0s0X4t7oswVX~HRS3~Vn4iRer3e6LQipw3Bm4IlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal