Abstract
Targeting dendritic cell (DC) functions such as migration is a pivotal mechanism used by HIV-1 to disseminate within the host. The HIV-1 envelope protein is the most important of the virally encoded proteins that exploits the migratory capacity of DCs. In the present study, we elucidated the signaling machinery involved in migration of immature DCs (iDCs) in response to HIV-1 envelope protein. We observed that M-tropic HIV-1 glycoprotein 120 (gp120) induces phosphorylation of the nonreceptor tyrosine kinase, Pyk2. Inhibition of Pyk2 activity using a pharmacologic inhibitor, kinase-inactive Pyk2 mutant, and Pyk2-specific small interfering RNA blocked gp120-induced chemotaxis, confirming the role of Pyk2 in iDC migration. In addition, we also illustrated the importance of Pyk2 in iDC migration induced by virion-associated envelope protein, using aldithriol-2–inactivated M-tropic HIV-1 virus. Further analysis of the downstream signaling mechanisms involved in gp120-induced migration revealed that Pyk2 activates p38 mitogen-activated protein kinase, which in turn activates the F-actin–binding protein, leukocyte-specific protein 1, and enhances its association with actin. Taken together, our studies provide an insight into a novel gp120-mediated pathway that regulates DC chemotaxis and contributes to the dissemination of HIV-1 within an infected person.
Introduction
Primary HIV-1 infections most commonly occur at the mucosal surfaces of the human body, where immature dendritic cells (iDCs) reside.1 The iDCs of the skin and mucosa have been implicated as the first cellular targets for HIV-1 after sexual contact.2 These HIV-infected iDCs cross the mucosal surfaces and facilitate transmission of the virus to CD4+ T cells in the lymphoid tissue.3,4 The migratory nature of DCs, along with their ability to recruit numerous T cells to the lymphoid tissue, identifies them as strong candidates in spreading HIV-1 within the host.5
Chemokines are key components in regulating the migration of immature and mature dendritic cells.6 iDCs express CCR5, which allows the DCs to migrate to the sites of inflammation where its ligands macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-3α, and RANTES are produced.7 Mature DCs, in contrast, down-regulate CCR5 but up-regulate CXCR4, and show enhanced chemotaxis toward CXCL12 (stromal-derived factor 1α).7 The chemokine receptors CCR5 and CXCR4 also act as coreceptors for macrophage-tropic (M-tropic) and T cell–tropic (T-tropic) HIV-1, respectively. The HIV-1 envelope protein has been shown modulate various DC functions such as maturation, cytokine production, T-cell stimulatory function, survival, and migration.8-10 M-tropic HIV-1 envelope has been shown to induce intracellular signals and modulate iDC migration by binding to CCR5 receptor.10 However, very little is known about the mechanisms that regulate migration of DCs in response to the HIV-1 envelope.
Pyk2 is a member of the nonreceptor protein tyrosine kinase family, related to focal adhesion kinase (FAK).11 However, unlike FAK, Pyk2 exhibits a more restricted tissue expression pattern and is primarily present in epithelial cells, neuronal cells, fibroblasts, hematopoietic cells, and endothelial cells.12-14 It is fast emerging as a critical “platform kinase” that couples several receptors, including chemokine receptors with a variety of downstream effectors, thus regulating various functions such as cell adhesion, proliferation, and most importantly, migration.11 Previous studies from our laboratory and others have underscored the potential relevance of Pyk2 activation in chemokine and HIV-1 glycoprotein 120 (gp120)–induced CCR5-mediated signaling events in T cells and macrophages.15-17 However, very little is known about its role in dendritic cells. The present study demonstrates a key role for Pyk2 in gp120-induced DC chemotaxis by regulating a novel signaling pathway sequentially activating the p38 mitogen-activated protein (MAP) kinase and the leukocyte-specific protein, LSP1.
LSP1 (also known as WP34, pp52, and leufactin) is a 53-kDa F-actin–binding phospho-protein expressed in all human leukocytes and leukocytic cell lines.18 LSP1 functions by polarizing and remodeling the actin cytoskeleton, thus modulating the motility of various leukocytes.19-21 Although LSP1 has been shown to regulate leukocyte migration in response to different stimuli,19-21 its role in DC migration is not known. Our results suggest that LSP1 activation and LSP1-actin association mediated by gp120 are important steps in inducing DC chemotaxis.
Our results suggest that HIV-1 gp120 induces a novel Pyk2-dependent signaling pathway that mediates DC migration and thus facilitates the dissemination of HIV-1 within the host.
Methods
Preparation of dendritic cells from blood
CD14+ monocytes were isolated from the buffy coats of healthy donors (American Red Cross) using human CD14-negative selection kit (StemCell Technologies) as per the manufacturer's protocol. Because only cells are used and are without identifiers, this work is exempt from institutional review board review. iDCs were derived from monocytes cultured for 6 days in RPMI media (10% fetal bovine serum) supplemented with 50 ng/mL human granulocyte-macrophage colony-stimulating factor and human interleukin-4 (PeproTech), as described previously.22
Antibodies and reagents
Fluorescein isothiocyanate (FITC)–conjugated antibodies CD1a (clone HI149), CD14, and CCR5; phycoerythrin (PE)–conjugated antibodies DC-SIGN and CXCR4; allophycocyanin (APC)–conjugated HLA-DR and CD4; as well as the isotype controls were obtained from BD Biosciences. The phospho-Pyk2 antibodies were obtained from Biosource; the total Pyk2 antibody, from BD Transduction Laboratories; the phospho-p38 MAP kinase, p38 MAP kinase, and actin antibodies, from Cell Signaling; the phospho–extracellular signal-related kinase (ERK), and glyceraldehyde phosphate dehydrogenase antibody, from Santa Cruz Biotechnology; and the anti-CCR5 neutralizing antibody (clone 45531), from R&D Systems. The chemokine RANTES was obtained from PeproTech, whereas the recombinant M-tropic gp120 (YU2 and ADA) and T-tropic gp120 (IIIB) were obtained from Immunodiagnostics. The pharmacologic inhibitors (tyrphostin A9, SB203580, SB220025, and PD98059) were obtained from Calbiochem.
Cell surface staining and flow cytometry analysis
To detect surface receptor expression, the iDCs were stained with the respective conjugated monoclonal antibodies, in addition to the isotype control. Flow cytometry was carried out using a FACSCalibur cytometer and the data were analyzed by CellQuest software (BD Biosciences).
Western blot analysis
Equivalent amounts of protein extracts were run on a 4% to 12% gradient acrylamide gel (NuPAGE Bis-Tris gel; Invitrogen) and transferred onto nitrocellulose membranes. Immunodetection involved specific primary antibodies, appropriate secondary antibodies conjugated to horseradish peroxidase, and the enhanced chemiluminescence Western blotting detection system (Amersham Biosciences).
Recombinant adeno-associated virus transduction
High-efficiency gene delivery of the wild-type Pyk2 and the dominant-negative Pyk2 mutant, Pyk2K457A (Pyk2MT), was accomplished using a recombinant adeno-associated virus (rAAV)–based method. The AAV vectors were prepared as described previously.23 Briefly, the Pyk2 constructs were inserted into a standard AAV vector plasmid, pACP, and packaged in AAV5 virions. Cell lysates were collected 2 days after transfection, and the virions isolated. The vector preps were then dialyzed before use. Genomic titers of the preparations, as determined by real-time polymerase chain reaction were about 1011 copies/mL. Immature DCs were transduced by application of the AAV in a minimal amount of serum-free medium for 3 hours at 37°C. RPMI containing 20% serum was then added to the cells to achieve a final serum concentration of 10%. The cells were finally incubated for 48 hours, after which they were used in migration assays or lysed for Western blot analysis.
siRNA-mediated knockdown of Pyk2
RNA interference–mediated knockdown of Pyk2 and LSP1 was performed using SMARTpool duplex RNA oligonucleotides obtained from Dharmacon. A nontargeting small interfering RNA (QIAGEN) was used as the control. iDCs were transfected with the siRNA using nucleofection (Amaxa Biosystems) according to the manufacturer's instructions.
Chemotaxis assays
Approximately 5 × 105 immature dendritic cells were added to the upper compartment of 24-well Transwell chambers with a pore size of 5 μm (Costar Corp). Medium (0.6 mL) with or without M-tropic gp120 was added to the lower compartment. The chambers were incubated for 3 hours at 37°C in 5% CO2. The cells in the lower compartment were counted on a hemocytometer.
Aldrithiol-2 inactivation of HIV-1 virus isolates
Frozen viral stocks were quickly thawed at 37°C in a water bath and treated with aldrithiol-2 (AT-2; Sigma) at a concentration of 1 mM for 1 hour at 37°C to inactivate the virus.24 AT-2 was then removed by ultrafiltration using a centrifugal filter device with a 100-kDa cutoff (Amicon Ultra; Millipore) by 3 changes of media, 12 mL each. Filtration was done at 4°C and more than 100-fold dilution of AT-2 was achieved. In parallel, AT-2–treated media were also filtered similarly to check effects of residual AT-2 on dendritic cells.
Confocal microscopy
Cells were cultured in chamber slides. Then slides were fixed in 4% paraformaldehyde for 15 minutes at room temperature and blocked with 5% normal goat serum in phosphate-buffered saline (PBS)/Triton for 60 minutes. The cells were treated with monoclonal anti-LSP1 antibody (BD Biosciences) overnight at 4°C, washed thrice with PBS, and stained with Alexa Fluor 568–labeled anti–mouse immunoglobulin G (IgG) antibody (Molecular Probes) and/or Alexa Fluor 488 phalloidin for 2 hours. Subsequent to washing with PBS thrice, the slides were mounted using Prolong Gold antifade with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen), and then examined under a Zeiss 510 Meta confocal microscope. The pictures are acquired using LSM 510 software (Carl Zeiss).
Actin polymerization assay
Cells (106/mL) were treated with M-tropic gp120 (1.2 μg/mL) for various time points, washed twice with PBS, and treated with Alexa Fluor 488 phalloidin (Molecular Probes) for 2 hours. After washing thrice in PBS cells, the cells were analyzed using FACScan (Becton Dickinson).
Statistical analysis
Reported data are the means plus or minus SD of at least 3 independent experiments performed in duplicate or triplicate. The statistical significance was determined by the Student t test.
Results
M-tropic HIV-1 gp120 activates Pyk2 in iDCs
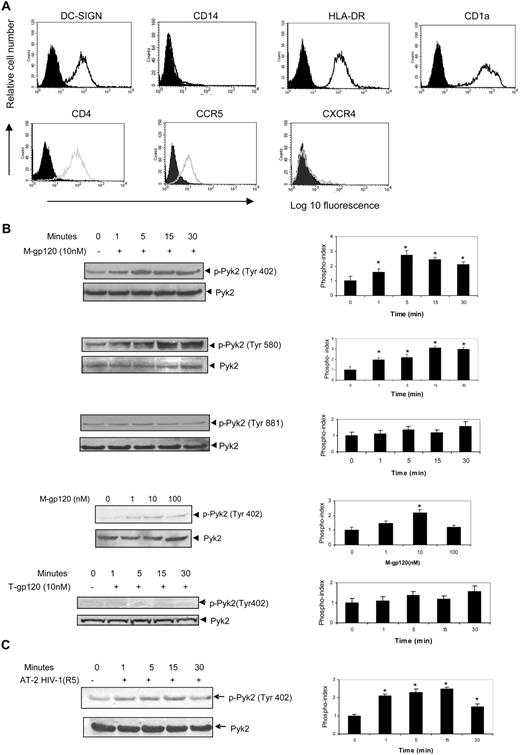
The iDCs were characterized on the sixth day of differentiation for the functional markers, DC-SIGN, CD14, HLA-DR, and CD1a by FACS analysis. iDCs showed a high expression of DC-SIGN, HLA-DR, and CD1a and minimal expression of CD14 (Figure 1A top panel). DCs were also tested for expression of the HIV receptor CD4 and coreceptors CXCR4 and CCR5. As expected, iDCs showed significant expression of CD4 and CCR5, but minimal expression of CXCR4 (Figure 1A bottom panel).
M-tropic HIV-1 envelope causes activation of Pyk2 in iDCs. (A) The surface expression of DC-SIGN, CD14, HLA-DR, CD1a, CD4, CCR5, and CXCR4 on immature dendritic cells was analyzed by flow cytometry. Monocyte-derived immature dendritic cells harvested on the sixth day of differentiation were stained with FITC-conjugated antibodies for CD14, CD1a, and CCR5; PE-conjugated antibodies for DC-SIGN and CXCR4; and APC-conjugated antibodies for HLA-DR and CD4. Cells were also stained with FITC-, PE-, or APC-conjugated isotype antibody controls. Filled peaks indicate antibody control and open peaks indicate receptor expression. (B) iDCs were stimulated with M-tropic gp120 (10 nM) for the indicated periods of time at 37°C. The lysates were analyzed by Western blot analysis using antibodies to phospho-Pyk2 (Tyr 402; first panel), phospho-Pyk2 (Tyr 580; second panel), and phospho-Pyk2 (Tyr 881; third panel). The same blot was then probed with total Pyk2 antibody. iDCs were also stimulated with various concentrations of M-tropic gp120 (0-100 nM) for 15 minutes at 37°C. The lysates were analyzed by Western blot analysis using antibodies specific to phospho-Pyk2 (Tyr 402; fourth panel). iDCs were stimulated with T-tropic gp120 (10 nM) for the indicated periods of time at 37°C and lysates analyzed by Western blot analysis using antibodies specific to phospho-Pyk2 (Tyr 402; fifth panel). The phosphorylation indices of the respective blots are shown at the right of the panels. For quantitative analysis of protein phosphorylation, the ratio of phosphorylation versus total protein in each lane was obtained by densitometry. The phosphorylation index was determined by calculating the value of this ratio in each lane and presenting the ratio as the fold increase over the control value (unstimulated sample; 0), which was designated as 1. *P < .05 versus the unstimulated control. Values on the right panel are mean ± SD of 3 independent experiments. Data on the left panel show one representative experiment of 3 independent experiments. (C) iDCs were stimulated with AT-2–inactivated HIV-1 (YU2; 3 μg/mL p24) for the indicated periods of time at 37°C. The lysates were analyzed by Western blot analysis using antibodies to phospho-Pyk2 (Tyr 402; left panel). The same blot was then probed with total Pyk2 antibody. The phosphorylation index of the blot is shown in the right panel. *P < .05 versus the unstimulated control. Values on the right panel are mean ± SD of 3 independent experiments. Data on left panel show 1 representative experiment of 3 independent experiments.
M-tropic HIV-1 envelope causes activation of Pyk2 in iDCs. (A) The surface expression of DC-SIGN, CD14, HLA-DR, CD1a, CD4, CCR5, and CXCR4 on immature dendritic cells was analyzed by flow cytometry. Monocyte-derived immature dendritic cells harvested on the sixth day of differentiation were stained with FITC-conjugated antibodies for CD14, CD1a, and CCR5; PE-conjugated antibodies for DC-SIGN and CXCR4; and APC-conjugated antibodies for HLA-DR and CD4. Cells were also stained with FITC-, PE-, or APC-conjugated isotype antibody controls. Filled peaks indicate antibody control and open peaks indicate receptor expression. (B) iDCs were stimulated with M-tropic gp120 (10 nM) for the indicated periods of time at 37°C. The lysates were analyzed by Western blot analysis using antibodies to phospho-Pyk2 (Tyr 402; first panel), phospho-Pyk2 (Tyr 580; second panel), and phospho-Pyk2 (Tyr 881; third panel). The same blot was then probed with total Pyk2 antibody. iDCs were also stimulated with various concentrations of M-tropic gp120 (0-100 nM) for 15 minutes at 37°C. The lysates were analyzed by Western blot analysis using antibodies specific to phospho-Pyk2 (Tyr 402; fourth panel). iDCs were stimulated with T-tropic gp120 (10 nM) for the indicated periods of time at 37°C and lysates analyzed by Western blot analysis using antibodies specific to phospho-Pyk2 (Tyr 402; fifth panel). The phosphorylation indices of the respective blots are shown at the right of the panels. For quantitative analysis of protein phosphorylation, the ratio of phosphorylation versus total protein in each lane was obtained by densitometry. The phosphorylation index was determined by calculating the value of this ratio in each lane and presenting the ratio as the fold increase over the control value (unstimulated sample; 0), which was designated as 1. *P < .05 versus the unstimulated control. Values on the right panel are mean ± SD of 3 independent experiments. Data on the left panel show one representative experiment of 3 independent experiments. (C) iDCs were stimulated with AT-2–inactivated HIV-1 (YU2; 3 μg/mL p24) for the indicated periods of time at 37°C. The lysates were analyzed by Western blot analysis using antibodies to phospho-Pyk2 (Tyr 402; left panel). The same blot was then probed with total Pyk2 antibody. The phosphorylation index of the blot is shown in the right panel. *P < .05 versus the unstimulated control. Values on the right panel are mean ± SD of 3 independent experiments. Data on left panel show 1 representative experiment of 3 independent experiments.
Pyk2 is a nonreceptor tyrosine kinase related to FAK that is expressed mainly in cells of hematopoietic and neuronal lineage.12,25,26 Pyk2 is a critical molecule in chemokine-receptor mediated signaling pathways in several types of cells.14,16,17,27,28 We therefore examined whether stimulation of iDCs with M-gp120 modulates the activity of Pyk2. Pyk2 has also been shown to be phosphorylated upon activation at 3 main tyrosine residues, Tyr 402, 579/580, and 881. We treated iDCs with 10 nM recombinant gp120 (1.2 μg/mL) from the M-tropic isolate (YU2) for different time periods and analyzed the levels of tyrosine phosphorylation of Pyk2 at these residues using specific antibodies. We observed that gp120 enhanced the tyrosine phosphorylation of residue 402 (2.7-fold), which is the major autophosphorylation site (Figure 1B first panel). There was also an increase in phosphorylation at Tyr 580 (3.1-fold), which is the kinase activation site (Figure 1B second panel). However, gp120 did not markedly induce the tyrosine phosphorylation of residue 881, which is the Grb2-binding site (Figure 1B third panel). These blots were reprobed with anti-Pyk2 antibody to confirm equal loading as well as expression of Pyk2 in these samples, as shown at the bottom of each subpanel (Figure 1B). M-tropic gp120 was shown to induce a concentration-dependent tyrosine phosphorylation of Pyk2 with maximal phosphorylation observed at 10 nM (2.2-fold; Figure 1B fourth panel). In addition, we also evaluated the activation of Pyk2 in response to RANTES, a cognate ligand of CCR5. In comparison with gp120, RANTES showed minimal activation of Pyk2 (data not shown), suggesting an alternate pathway in RANTES-induced CCR5 signaling. Although iDCs derived from monocytes of most donors demonstrated a robust response to gp120 stimulation, there was some donor to donor variability in the level of constitutive Pyk2 phosphorylation under unstimulated conditions and the magnitude of phosphorylation response to gp120. We did not observe any significant phosphorylation of Pyk2 with T-tropic gp120 (HIV-1 IIIB), likely due to the minimal CXCR4 expression in iDCs (Figure 1B fifth panel). We have also shown that pertussis toxin does not inhibit M-gp120–induced activation of Pyk2 (data not shown), suggesting that Pyk2 tyrosine kinase activation is independent of Gi-coupled receptors.
HIV-1 surface envelope glycoproteins are produced in 2 forms in infected persons: free monomeric gp120 and trimeric gp120 present at the surface of the virions. Because the conformation of trimeric gp120 is known to differ from the monomeric form, we investigated whether virion-bound trimeric gp120 could also induce activation of Pyk2 in iDCs. We used AT-2–inactivated HIV-1 YU2 virions, which harbor intact envelope glycoproteins, but are unable to initiate productive infection due to covalent modification of nucleocapsid proteins. These inactivated virions are competent for entry, but are blocked in later steps of the viral life cycle, which makes them useful to assess envelope-mediated events. We observed that AT-2–inactivated virions significantly activated Pyk2 (2.3-fold; Figure 1C) versus the control preparation. The results in Figure 1B and C indicate that gp120, in both its monomeric and trimeric form, induces Pyk2 activation in iDCs.
M-tropic HIV-1 gp120 stimulation of iDCs facilitates chemotaxis through the CCR5 chemokine receptor
We next addressed the question of whether gp120-induced Pyk2 activation in iDCs results in functional consequences with potential biologic significance. HIV-1 exposure or infection induces a variety of functional and secretory responses in iDCs including migration, cytokine production, and immune activation.10,29,30 Pyk2 is a kinase shown to play an important role in regulating chemotaxis of different types of immune cells in response to integrin and chemokine receptors.27,31,32 We therefore investigated the involvement of Pyk2 in gp120-induced chemotaxis in iDCs.
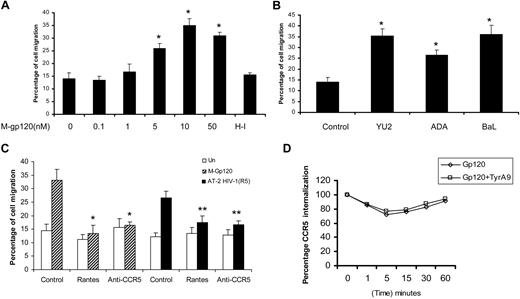
First, we standardized the migration of monocyte-derived iDCs in response to various concentrations of gp120 from the M-tropic HIV-1 strain, YU2 (Figure 2A). A previous study has demonstrated the migration of iDCs toward M-tropic HIV-1 culture supernatants as well as recombinant M-tropic gp120 (BaL).10 In agreement with the previous study, we also found that M-tropic gp120 induced enhanced migration of immature dendritic cells, compared with the untreated and heat-inactivated (HI) control. The chemotactic effect of M-gp120 was present over a range of concentrations with maximal chemotaxis (35%) occurring at 10 nM (Figure 2A). We compared DC migration using gp120 from different strains of M-tropic HIV-1, YU2, ADA, and BaL (Figure 2B). ADA showed slightly lower levels of migration (25%), compared with YU2 (35%) and BaL (36%). Hence, we used HIV-1 gp120 (YU2) for the rest of the studies.
M-tropic HIV envelope induces migration of iDCs via CCR5. (A) The ability of monocyte-derived iDCs to migrate in response to various concentrations of HIV-1 M-tropic gp120 (0-50 nM) and the heat-inactivated (HI) control was analyzed using transwell migration assays. *P < .05 versus the heat-inactivated control. (B) Migration of iDCs in response to various clones of M-tropic gp120, YU2, ADA, and BaL (10 nM) was determined using transwell assays. *P < .05 versus the untreated control. (C) iDCs were pretreated with anti-CCR5 neutralizing antibodies (10 μg/mL) or RANTES (100 nM) for 1 hour at 37°C. The cells were then used in transwell migration assays in response to M-tropic gp120 (10 nM) and AT-2 HIV-1 (used at a final concentration of 3 μg p24/mL). *P < .05 versus the gp120-treated control. **P < .05 versus the AT-2 HIV-treated control. Data represent mean ± SD of 3 independent experiments. (D) iDCs were treated with tyrphostin (5 μM) or vehicle alone at 37°C for 1 hour and subsequently incubated with medium alone or M-gp120 (100 nM) at 37°C for different periods of time. After washing, cells were stained with control mouse IgG or monoclonal CCR5-FITC antibody. The percentage of cells positively stained with anti-CCR5 Ab was determined by flow cytometry. The graph shows a comparison of the percentage of internalization between the tyrphostin A9–treated and vehicle-treated iDCs stimulated with M-gp120. Results are representative of 3 separate experiments.
M-tropic HIV envelope induces migration of iDCs via CCR5. (A) The ability of monocyte-derived iDCs to migrate in response to various concentrations of HIV-1 M-tropic gp120 (0-50 nM) and the heat-inactivated (HI) control was analyzed using transwell migration assays. *P < .05 versus the heat-inactivated control. (B) Migration of iDCs in response to various clones of M-tropic gp120, YU2, ADA, and BaL (10 nM) was determined using transwell assays. *P < .05 versus the untreated control. (C) iDCs were pretreated with anti-CCR5 neutralizing antibodies (10 μg/mL) or RANTES (100 nM) for 1 hour at 37°C. The cells were then used in transwell migration assays in response to M-tropic gp120 (10 nM) and AT-2 HIV-1 (used at a final concentration of 3 μg p24/mL). *P < .05 versus the gp120-treated control. **P < .05 versus the AT-2 HIV-treated control. Data represent mean ± SD of 3 independent experiments. (D) iDCs were treated with tyrphostin (5 μM) or vehicle alone at 37°C for 1 hour and subsequently incubated with medium alone or M-gp120 (100 nM) at 37°C for different periods of time. After washing, cells were stained with control mouse IgG or monoclonal CCR5-FITC antibody. The percentage of cells positively stained with anti-CCR5 Ab was determined by flow cytometry. The graph shows a comparison of the percentage of internalization between the tyrphostin A9–treated and vehicle-treated iDCs stimulated with M-gp120. Results are representative of 3 separate experiments.
In addition, we investigated whether virion-associated trimeric gp120 could induce chemotaxis. AT-2 HIV-1 virions (3 μg p24 Gag/mL) promoted iDC cell migration (29%) in comparison with the control (12%; Figure 2C). Further, we investigated whether the chemokine receptor CCR5 was responsible for HIV-1 envelope–mediated chemotaxis. To probe CCR5, we used the CCR5 neutralizing antibody as well as its cognate ligand, RANTES. Migration assays indicated that treatment with anti-CCR5 antibody, as well as RANTES, inhibited M-gp120–induced migration by 88% and 95%, respectively. Preincubation of iDCs with RANTES and anti-CCR5 antibody also abrogated AT-2 HIV-1–mediated migration by 71% and 72%, respectively (Figure 2C). These findings suggest that M-tropic gp120–mediated chemotaxis in iDCs is specifically mediated by the envelope glycoproteins' cognate coreceptor, CCR5. We next investigated whether gp120-induced down-regulation of CCR5 was regulated by Pyk2 phosphorylation. As shown in Figure 2D, the CCR5 expression on the cell surface, as analyzed by flow cytometry, was significantly down-regulated upon M-gp120 treatment (100 nM). However, pretreatment of the cells with tyrphostin A9 (5 μM) at 37°C for 1 hour did not inhibit the gp120-induced CCR5 down-regulation on the cell membrane, suggesting that M-gp120–mediated CCR5 down-regulation may not involve Pyk2 tyrosine kinase activation.
Pyk2 modulates gp120-induced iDC migration
To elucidate the importance of Pyk2 in gp120-induced migration of DCs, we used pharmacologic inhibitors, dominant-negative constructs, as well as siRNA, specific to Pyk2.
Tyrphostin A9 blocks gp120-induced dendritic cell migration.
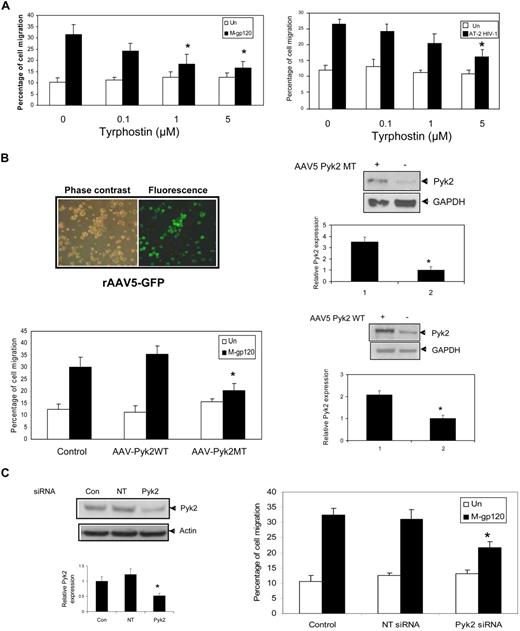
We first investigated the effect of putative pharmacologic Pyk2 inhibitor, tyrphostin A9 (Tyr A9), to analyze the functional significance of gp120-induced phosphorylation. Tyrphostin A9 is an extremely effective tool for investigating the role of Pyk2 in cellular signaling. In a study involving tumor necrosis factor alpha–stimulated neutrophils,25 tyrphostin A9 was the most selective of 51 tyrosine kinase inhibitors in blocking the Pyk2 signaling pathway. iDCs left untreated, pretreated with different concentrations of Tyr A9 (0.1, 1, and 5 μM), or pretreated with dimethyl sulfoxide control for 1 hour were used in chemotaxis assays in response to M-tropic gp120. The Pyk2 inhibitor–treated cells showed significant decrease in migration compared with vehicle-treated control (Figure 3A left panel). Nearly 80% inhibition of chemotaxis was observed at 5 μM Tyr A9 concentrations.
Inhibition of Pyk2 activation attenuates M-tropic gp120–induced iDC migration. (A) iDCs were pretreated with vehicle (dimethyl sulfoxide) or tyrphostin A9 (5 μM) for 1 hour at 37°C. The migration of these cells in response to M-tropic gp120 (10 nM) (left panel) or AT-2–inactivated virions (3 μg/mL p24) was determined after 3 hours of incubation. *P < .05 versus the vehicle control. (B) iDCs infected with rAAV5-expressing GFP protein were analyzed for GFP expression 48 hours after infection (top left panel) using a Zeiss Axiovert 40 CFL microscope (40×/0.50 NA). The fluorescence micrographs are shown on the right, whereas the corresponding phase-contrast image is shown on the left of the panel. iDCs were then transduced with recombinant AAV5-expressing Pyk2 mutant (Pyk2MT) or Pyk2 wild-type (Pyk2WT). Overexpression of the mutant and wild-type Pyk2 was demonstrated by Western blot analysis with anti-Pyk2 antibodies 48 hours after transduction. (Top and bottom right panels) Anti–glyceraldehyde phosphate dehydrogenase antibody was used as an internal control. The bar graph at the bottom of the panels shows the quantitative analysis of Pyk2 expression obtained by densitometry. *P < .05 versus the vector control. Data represent mean ± SD of 3 independent experiments. The transduced cells were tested for their ability to migrate in response to M-tropic gp120 (10 nM) using the chemotaxis assay (bottom left panel). *P < .05 versus the M-tropic gp120–treated vector control. Data represent mean ± SD of 3 independent experiments. (C) iDCs were transfected with Pyk2-specific siRNA and nontargeting siRNA (NT siRNA) using nucleofection (Amaxa Biosystems). The knockdown of Pyk2 expression was analyzed by Western blot analysis with anti-Pyk2 antibodies (left panel). Antiactin antibody served as a control. The bar graph at the bottom of the panel shows the quantitative analysis of Pyk2 expression obtained by densitometry. *P < .05 versus the nontargeting vector control. Data represent mean ± SD of 3 independent experiments. The siRNA-transfected iDCs were used in the transwell migration assay in response to gp120 (10 nM; right panel). *P < .05 versus the M-tropic gp120–treated nontargeting siRNA control. Data represent mean ± SD of 3 independent experiments.
Inhibition of Pyk2 activation attenuates M-tropic gp120–induced iDC migration. (A) iDCs were pretreated with vehicle (dimethyl sulfoxide) or tyrphostin A9 (5 μM) for 1 hour at 37°C. The migration of these cells in response to M-tropic gp120 (10 nM) (left panel) or AT-2–inactivated virions (3 μg/mL p24) was determined after 3 hours of incubation. *P < .05 versus the vehicle control. (B) iDCs infected with rAAV5-expressing GFP protein were analyzed for GFP expression 48 hours after infection (top left panel) using a Zeiss Axiovert 40 CFL microscope (40×/0.50 NA). The fluorescence micrographs are shown on the right, whereas the corresponding phase-contrast image is shown on the left of the panel. iDCs were then transduced with recombinant AAV5-expressing Pyk2 mutant (Pyk2MT) or Pyk2 wild-type (Pyk2WT). Overexpression of the mutant and wild-type Pyk2 was demonstrated by Western blot analysis with anti-Pyk2 antibodies 48 hours after transduction. (Top and bottom right panels) Anti–glyceraldehyde phosphate dehydrogenase antibody was used as an internal control. The bar graph at the bottom of the panels shows the quantitative analysis of Pyk2 expression obtained by densitometry. *P < .05 versus the vector control. Data represent mean ± SD of 3 independent experiments. The transduced cells were tested for their ability to migrate in response to M-tropic gp120 (10 nM) using the chemotaxis assay (bottom left panel). *P < .05 versus the M-tropic gp120–treated vector control. Data represent mean ± SD of 3 independent experiments. (C) iDCs were transfected with Pyk2-specific siRNA and nontargeting siRNA (NT siRNA) using nucleofection (Amaxa Biosystems). The knockdown of Pyk2 expression was analyzed by Western blot analysis with anti-Pyk2 antibodies (left panel). Antiactin antibody served as a control. The bar graph at the bottom of the panel shows the quantitative analysis of Pyk2 expression obtained by densitometry. *P < .05 versus the nontargeting vector control. Data represent mean ± SD of 3 independent experiments. The siRNA-transfected iDCs were used in the transwell migration assay in response to gp120 (10 nM; right panel). *P < .05 versus the M-tropic gp120–treated nontargeting siRNA control. Data represent mean ± SD of 3 independent experiments.
We next investigated whether virion-bound trimeric gp120 could also induce chemotaxis through the Pyk2-dependent pathway. As shown in Figure 3A right panel, AT-2–inactivated virion-induced chemotaxis was blocked by tyrphostin A9 (62% inhibition), indicating that AT-2–inactivated HIV-1–induced iDC migration is also mediated through Pyk2. The above results indicate that gp120 in both its monomeric, as well as its trimeric form, induced Pyk2 activation leading to enhanced chemotaxis.
Pyk2 mutant protein inhibits gp120-induced migration.
To further confirm the role of Pyk2 in gp120-induced DC migration, we studied the effect of recombinant AAV-expressing wild-type Pyk2 (Pyk2WT) and a kinase inactive mutant (Pyk2MT) on the migration of iDCs. Pyk2MT contains a mutation of lysine 457 to alanine in the tyrosine-kinase domain of Pyk2 that results in generation of catalytically inactive Pyk2.
First, we optimized the transduction efficiency of the AAV vectors into dendritic cells using the AAV5–green fluorescent protein (GFP) construct (Figure 3B top left panel). We achieved a mean of 93% efficiency in transduction of AAV-GFP into immature DCs. In our experiments, we evaluated both rAAV-2 and rAAV-5 to transduce iDCs. The percentage of cells transduced using rAAV5-GFP (90%-95%) was much higher compared with rAAV2-GFP (60%-65%; data not shown). Expression of wild-type and mutant Pyk2 proteins in DCs was confirmed by Western blotting (Figure 3B right panel). Next, we examined the effect of infection with the wild-type AAV-Pyk2 and AAV-Pyk2MT on cell migration in response to gp120. Our results indicated that the iDCs transduced with the catalytically inactive Pyk2 mutant (AAV-Pyk2MT) significantly attenuated M-tropic gp120–induced migration by 76%, compared with the control (Figure 3B bottom left panel), as well as wild-type Pyk2 (AAV-Pyk2WT). These results provide direct evidence for the role of Pyk2 in M-tropic gp120–induced migration.
Inhibition of Pyk2 activity using specific siRNA inhibits M-gp120–induced migration.
We also used specific Pyk2 siRNAs and transfected them into iDCs using the nucleofector device (Amaxa Biosystems). Our optimization studies indicated that the transfection with siRNA Pyk2 (100 nM) specifically decreased expression of Pyk2 by 50% to 55% in comparison with transfection with a nontargeting siRNA (Figure 3C left panel). The Pyk2-siRNA–treated iDCs showed significantly lower migration in response to gp120 (9%) compared with iDCs treated with nontargeting siRNA control (22%; Figure 3C right panel). Taken together, these data suggest that Pyk2-dependent activation is an important step in gp120-mediated chemotaxis.
p38 MAP kinase and LSP-1 are critical downstream effectors of Pyk2 that mediate gp120-induced chemotaxis
p38 MAP kinase activation.
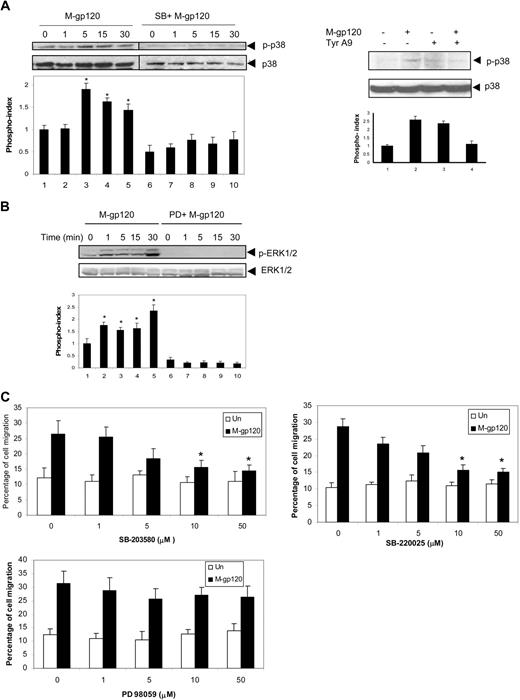
To address the downstream consequences of gp120-induced Pyk2 activation leading to migration, we next focused on the MAP kinase family of signaling molecules. Among the MAP kinase family members, the p38 MAP kinase and ERK kinases have been demonstrated to be important downstream effectors of Pyk2 in a variety of cells.33,34 Hence we looked at the phosphorylation of these kinases in response to gp120. iDCs were stimulated with gp120 and cells analyzed by Western blot using polyclonal antibodies specific for phospho-ERK1/2 and phospho-p38 MAP kinase. Stimulation with gp120 resulted in robust phosphorylation of p38 MAP kinase (Figure 4A left panel), and was maximal at 5 (2-fold) to 15 (1.7-fold) minutes. Gp120-induced p38 MAPK phosphorylation was blocked by the specific p38 MAPK inhibitor, SB-203580 (Figure 4A), thus confirming the specificity of the inhibitor. Furthermore, we also showed that inhibition of Pyk2 activity using tyrphostin A9 inhibited gp120-induced p38 phosphorylation (Figure 4A right panel), indicating that p38 MAP kinase functions downstream of Pyk2. HIV-1 gp120 also induced phosphorylation of ERK kinase, which was blocked by the specific ERK kinase inhibitor, PD98059 (Figure 4B), thus confirming the specificity of the inhibitor. However, gp120-induced ERK phosphorylation was not attenuated using tyrphostin A9 (data not shown). The involvement of the p38 MAP kinase pathway in gp120-induced chemotaxis was confirmed by the dose-dependent blocking of the migration with a specific inhibitor of p38 MAP kinase, SB203580 (Figure 4C top left panel), as well as SB220025 (Figure 4C top right panel). SB203580 showed an inhibition of 65% at 10 μM and 75% at 50 μM, whereas SB220025 showed an inhibition of 72% at 10 μM and 80% at 50 μM concentration. However, pretreatment with the specific ERK kinase inhibitor, PD98059, had a partial effect on gp120-induced iDC chemotaxis (Figure 4C bottom panel). PD98059 showed an inhibition of 25% at 10 μM and 34% at 50 μM concentration. These results suggest that p38 MAP kinase may be a more important component compared with ERK kinase in the gp120-induced signaling pathway that regulates DC migration. Furthermore, these results also indicate that gp120 stimulates chemotaxis through a p38 MAP kinase–dependent pathway, which is downstream of Pyk2.
p38 MAP kinase mediates gp120-induced iDC chemotaxis downstream of Pyk2. (A) iDCs were pretreated with SB203589 (10 μM) for 1 hour at 37°C and stimulated with gp120 (10 nM) at 37°C for various periods of time. The cells were lysed and analyzed by Western blotting with anti–phospho-p38 antibodies (left panel). The same blots were reprobed with anti-p38 antibodies. iDCs were pretreated with vehicle or tyrphostin A9 (5 μM) for 1 hour at 37°C. The cells were then stimulated with HIV-1 gp120 for 15 minutes, lysed, and analyzed by Western blotting with anti–phospho-p38 antibody (right panel). The same blot was reprobed with anti-p38 antibody. The bar graph at the bottom of the panels represents the phosphorylation index as obtained by densitometry. *P < .05 versus the unstimulated control. Data represent mean ± SD of 3 independent experiments. (B) iDCs were pretreated with PD98059 (10 μM) for 1 hour at 37°C and stimulated with gp120 (10 nM) at 37°C for various periods of time. The cells were lysed and analyzed by Western blotting with anti–p-ERK1/2 antibodies. The same blots were reprobed with anti–ERK1/2 antibodies. Data show 1 representative experiment of 3 independent experiments. (C) iDCs were pretreated with vehicle or various concentrations of the p38 MAP kinase inhibitors, SB203580 (top left panel) and SB220025 (top right panel) as well as the ERK kinase inhibitor (PD98059; bottom panel) for 1 hour at 37°C. The migration of these cells in response to M-tropic gp120 (10 nM) was determined after 3 hours of incubation. *P < .05 versus the vehicle control.
p38 MAP kinase mediates gp120-induced iDC chemotaxis downstream of Pyk2. (A) iDCs were pretreated with SB203589 (10 μM) for 1 hour at 37°C and stimulated with gp120 (10 nM) at 37°C for various periods of time. The cells were lysed and analyzed by Western blotting with anti–phospho-p38 antibodies (left panel). The same blots were reprobed with anti-p38 antibodies. iDCs were pretreated with vehicle or tyrphostin A9 (5 μM) for 1 hour at 37°C. The cells were then stimulated with HIV-1 gp120 for 15 minutes, lysed, and analyzed by Western blotting with anti–phospho-p38 antibody (right panel). The same blot was reprobed with anti-p38 antibody. The bar graph at the bottom of the panels represents the phosphorylation index as obtained by densitometry. *P < .05 versus the unstimulated control. Data represent mean ± SD of 3 independent experiments. (B) iDCs were pretreated with PD98059 (10 μM) for 1 hour at 37°C and stimulated with gp120 (10 nM) at 37°C for various periods of time. The cells were lysed and analyzed by Western blotting with anti–p-ERK1/2 antibodies. The same blots were reprobed with anti–ERK1/2 antibodies. Data show 1 representative experiment of 3 independent experiments. (C) iDCs were pretreated with vehicle or various concentrations of the p38 MAP kinase inhibitors, SB203580 (top left panel) and SB220025 (top right panel) as well as the ERK kinase inhibitor (PD98059; bottom panel) for 1 hour at 37°C. The migration of these cells in response to M-tropic gp120 (10 nM) was determined after 3 hours of incubation. *P < .05 versus the vehicle control.
LSP1 activation.
LSP1 is an F-actin–binding phospho-protein recently shown to be a downstream substrate of p38 MAP kinase in neutrophils.35 It is expressed in all human leukocytes and endothelial cells.36 In leukocytes, LSP1 promotes actin polymerization and cytoskeletal remodeling, thus influencing cell motility.21,37 Hence we studied the activation of LSP1 in response to gp120 in DCs.
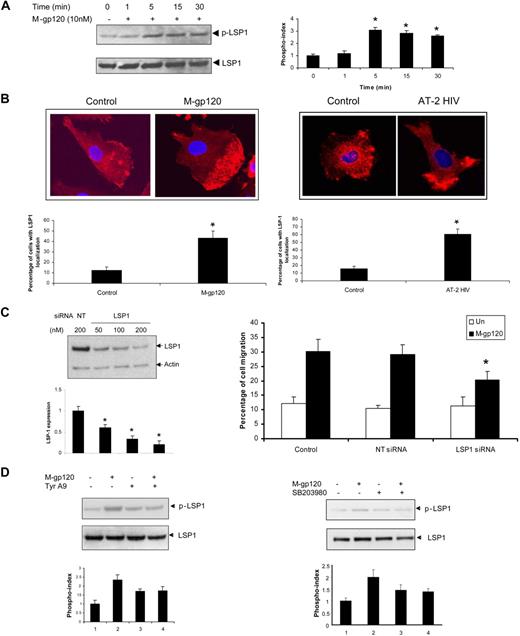
Because LSP1 is activated by phosphorylation of its serine residues, we first evaluated the serine phosphorylation of LSP1 in response to gp120. Phosphorylation of LSP1 was demonstrated using anti–phospho LSP1 (Ser 204) antibodies (Figure 5A). Reprobing of these blots with anti-LSP1 revealed that equal amounts of protein were present in each sample. These results clearly indicate that LSP1 is activated in response to gp120. Phosphorylation was maximal at 5 minutes (3-fold). Confocal microscopic studies showed that there was localization of LSP1 at the uropods upon treatment with both M-tropic gp120 (Figure 5B left panel) as well as AT-2–inactivated virus (Figure 5B right panel). To elucidate the role of LSP-1 in gp120-induced migration, we evaluated the effect of LSP1 knockdown in iDCs using specific siRNA in iDCs. Western blot analysis revealed a significant down-regulation (75%) of LSP-1 expression (Figure 5C left panel). When LSP1-siRNA–treated cells were subjected to gp120-induced chemotaxis, there was a significant attenuation (53%) of migration, compared with the cells treated with NT siRNA (Figure 5C right panel), confirming the role for LSP1 in gp120-induced DC migration.
Activation of the LSP1 downstream of Pyk2 and p38 MAP kinase mediates gp120-induced iDC chemotaxis. (A) iDCs were untreated (0) or stimulated with M-tropic gp120 (10 nM) for the indicated time points. The cells were lysed and analyzed by Western blotting with p-LSP1 (left panel) antibody. The blots were stripped and reprobed with anti-LSP1 antibody. The bar graph (right panel) represents the phosphorylation index as obtained by densitometry. *P < .05 versus the unstimulated control. Data represent mean ± SD of 3 independent experiments. (B) iDCs cultured in chambered slides were treated with M-tropic gp120 (10 nM; left panel) or AT-2 HIV-1 (right panel) for 30 minutes. The cells were then fixed, permeabilized, and treated with rabbit anti-LSP1antibody. After washing, cells were probed with Alexa 568–tagged anti–rabbit IgG antibody and the slides were mounted using Prolong Gold antifade with DAPI (Invitrogen), and then examined under a Zeiss confocal microscope (63×/1.4 oil). The pictures were acquired using LSM 510 software. (C) iDCs were transfected with LSP1-specific siRNA and nontargeting siRNA (NT siRNA) using nucleofection (Amaxa Biosystems). The knockdown of LSP1 expression was analyzed by Western blot analysis with anti-LSP1 antibodies (left panel). Antiactin antibody served as a control. The bar graph at the bottom of the panel shows the quantitative analysis of LSP1 expression obtained by densitometry. *P < .05 versus the nontargeting vector control. Data represent mean ± SD of 3 independent experiments. The siRNA-transfected iDCs were used in the transwell migration assay in response to gp120 (10 nM; right panel). *P < .05 versus the M-tropic gp120–treated nontargeting siRNA control. (D) iDCs were pretreated with vehicle or tyrphostin A9 (5 μM) (left panel) or p38 MAP kinase inhibitor, SB203580 (30 μM; right panel), for 1 hour at 37°C. The cells were then stimulated with gp120 (10 nM) for 30 minutes. The cells were lysed and analyzed by Western blotting with anti–phospho-LSP1 antibody. The same blots were probed with anti-LSP1 antibody. The bar graph at the bottom of the panels represents the phosphorylation index as obtained by densitometry. *P < .05 versus the unstimulated control. Data represent mean ± SD of 3 independent experiments. Data show one representative experiment of 3 independent experiments.
Activation of the LSP1 downstream of Pyk2 and p38 MAP kinase mediates gp120-induced iDC chemotaxis. (A) iDCs were untreated (0) or stimulated with M-tropic gp120 (10 nM) for the indicated time points. The cells were lysed and analyzed by Western blotting with p-LSP1 (left panel) antibody. The blots were stripped and reprobed with anti-LSP1 antibody. The bar graph (right panel) represents the phosphorylation index as obtained by densitometry. *P < .05 versus the unstimulated control. Data represent mean ± SD of 3 independent experiments. (B) iDCs cultured in chambered slides were treated with M-tropic gp120 (10 nM; left panel) or AT-2 HIV-1 (right panel) for 30 minutes. The cells were then fixed, permeabilized, and treated with rabbit anti-LSP1antibody. After washing, cells were probed with Alexa 568–tagged anti–rabbit IgG antibody and the slides were mounted using Prolong Gold antifade with DAPI (Invitrogen), and then examined under a Zeiss confocal microscope (63×/1.4 oil). The pictures were acquired using LSM 510 software. (C) iDCs were transfected with LSP1-specific siRNA and nontargeting siRNA (NT siRNA) using nucleofection (Amaxa Biosystems). The knockdown of LSP1 expression was analyzed by Western blot analysis with anti-LSP1 antibodies (left panel). Antiactin antibody served as a control. The bar graph at the bottom of the panel shows the quantitative analysis of LSP1 expression obtained by densitometry. *P < .05 versus the nontargeting vector control. Data represent mean ± SD of 3 independent experiments. The siRNA-transfected iDCs were used in the transwell migration assay in response to gp120 (10 nM; right panel). *P < .05 versus the M-tropic gp120–treated nontargeting siRNA control. (D) iDCs were pretreated with vehicle or tyrphostin A9 (5 μM) (left panel) or p38 MAP kinase inhibitor, SB203580 (30 μM; right panel), for 1 hour at 37°C. The cells were then stimulated with gp120 (10 nM) for 30 minutes. The cells were lysed and analyzed by Western blotting with anti–phospho-LSP1 antibody. The same blots were probed with anti-LSP1 antibody. The bar graph at the bottom of the panels represents the phosphorylation index as obtained by densitometry. *P < .05 versus the unstimulated control. Data represent mean ± SD of 3 independent experiments. Data show one representative experiment of 3 independent experiments.
Furthermore, pretreatment of iDCs with Pyk2 inhibitor, tyrphostin A9, inhibited HIV gp120-mediated LSP1 serine phosphorylation (Figure 5D left panel). LSP1 phosphorylation was also inhibited with the p38 MAP kinase inhibitor, SB203580 (Figure 5D right panel). These results confirm that LSP1 is modulated downstream of Pyk2 and p38 MAPK in iDCs in response to M-tropic gp120.
LSP1-actin association.
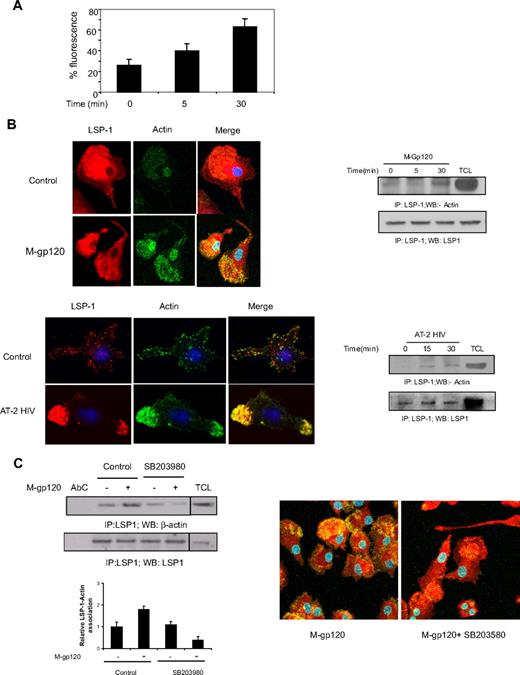
Because LSP1 is an F-actin–binding protein, we investigated its association with actin on stimulation with gp120. Actin is an important cytoskeletal protein that mediates the migration of various cell types.38 First, we confirmed the polymerization of actin in response to M-tropic gp120 (Figure 6A). Confocal microscopic study experiments revealed that stimulation with both M-tropic HIV gp120 as well as AT-2 HIV-1 significantly enhanced association between LSP1 and F-actin in iDCs (Figure 6B left panel). Immunoprecipitation studies also revealed an association between LSP1 and actin upon stimulation with both M-tropic gp120 and AT-2 HIV-1 (Figure 6B right panel). Furthermore, pretreatment of iDCs with the p38 MAP kinase inhibitor, SB203580, attenuated the LSP1-actin association. This was demonstrated both by immunoprecipitation (Figure 6C left panel) and confocal microscopy (Figure 6C right panel). These results confirm that M-tropic HIV gp120-induced activation of p38 MAP kinase mediates the phosphorylation of LSP1 and its association with actin.
Gp120 induces LSP-1–actin association downstream of p38 MAP kinase. (A) iDCs were untreated (0) or treated with M-tropic gp120 and stained with Alexa Fluor 488–phalloidin (Invitrogen) and the cells were analyzed by flow cytometry. (B) iDCs were cultured in chambered slides, and either left untreated (C or 0) and were treated with M-tropic gp120 (top left panel) or AT-2–inactivated HIV-1 (bottom left panel) for 30 minutes. Then cells were fixed and treated with rabbit anti–LSP-1antibody. After washing, cells were probed with Alexa 568–tagged anti–rabbit IgG antibody and Alexa Fluor 488–phalloidin. The slides were mounted using Prolong Gold antifade with DAPI (Invitrogen), and then examined under a Zeiss confocal microscope (63×/1.4 oil). The pictures were acquired using LSM 510 software. iDCs were unstimulated or stimulated with M-tropic gp120 (10 nM; top right panel) or AT-2 HIV (3 μg/mL p24; bottom right panel) for various time periods. The cells were lysed, and the lysates were immunoprecipitated with anti–LSP-1 antibody and immunoblotted with anti–β-actin antibody (top panel). The blots were stripped and reprobed with anti–LSP 1 antibody (bottom panel) to check the protein concentration in each lane. (C) iDCs were unstimulated or stimulated with M-tropic gp120 (10 nM) for 30 minutes in presence or absence of p38 inhibitor, SB203580 (30μM). The cells were lysed, and the lysates were immunoprecipitated with anti–LSP-1 antibody and Western blotted with anti–β-actin antibody (left panel). The blots were stripped and reprobed with anti–LSP-1 antibody to check the protein concentration in each lane. A vertical line has been inserted to indicate a repositioned gel lane. The bar graph at the bottom of the panel shows the quantitative analysis of LSP-1–actin association of the blots as obtained by densitometry.*P < .05 versus the unstimulated control. Data represent mean ± SD of 3 independent experiments. iDCs were cultured in chambered slides, then treated with M-tropic gp120 for 30 minutes in presence or absence of p38 inhibitor, SB203580 (30 μM). The cells were fixed and treated with rabbit anti–LSP-1 antibody. After washing, cells were probed with Alexa 568–tagged anti–rabbit IgG antibody and Alexa Fluor 488–phalloidin (right panel). The slides were mounted using Prolong Gold antifade with DAPI (Invitrogen), and then examined under a Zeiss confocal microscope (63×/1.4 oil). The pictures were acquired using LSM 510 software.
Gp120 induces LSP-1–actin association downstream of p38 MAP kinase. (A) iDCs were untreated (0) or treated with M-tropic gp120 and stained with Alexa Fluor 488–phalloidin (Invitrogen) and the cells were analyzed by flow cytometry. (B) iDCs were cultured in chambered slides, and either left untreated (C or 0) and were treated with M-tropic gp120 (top left panel) or AT-2–inactivated HIV-1 (bottom left panel) for 30 minutes. Then cells were fixed and treated with rabbit anti–LSP-1antibody. After washing, cells were probed with Alexa 568–tagged anti–rabbit IgG antibody and Alexa Fluor 488–phalloidin. The slides were mounted using Prolong Gold antifade with DAPI (Invitrogen), and then examined under a Zeiss confocal microscope (63×/1.4 oil). The pictures were acquired using LSM 510 software. iDCs were unstimulated or stimulated with M-tropic gp120 (10 nM; top right panel) or AT-2 HIV (3 μg/mL p24; bottom right panel) for various time periods. The cells were lysed, and the lysates were immunoprecipitated with anti–LSP-1 antibody and immunoblotted with anti–β-actin antibody (top panel). The blots were stripped and reprobed with anti–LSP 1 antibody (bottom panel) to check the protein concentration in each lane. (C) iDCs were unstimulated or stimulated with M-tropic gp120 (10 nM) for 30 minutes in presence or absence of p38 inhibitor, SB203580 (30μM). The cells were lysed, and the lysates were immunoprecipitated with anti–LSP-1 antibody and Western blotted with anti–β-actin antibody (left panel). The blots were stripped and reprobed with anti–LSP-1 antibody to check the protein concentration in each lane. A vertical line has been inserted to indicate a repositioned gel lane. The bar graph at the bottom of the panel shows the quantitative analysis of LSP-1–actin association of the blots as obtained by densitometry.*P < .05 versus the unstimulated control. Data represent mean ± SD of 3 independent experiments. iDCs were cultured in chambered slides, then treated with M-tropic gp120 for 30 minutes in presence or absence of p38 inhibitor, SB203580 (30 μM). The cells were fixed and treated with rabbit anti–LSP-1 antibody. After washing, cells were probed with Alexa 568–tagged anti–rabbit IgG antibody and Alexa Fluor 488–phalloidin (right panel). The slides were mounted using Prolong Gold antifade with DAPI (Invitrogen), and then examined under a Zeiss confocal microscope (63×/1.4 oil). The pictures were acquired using LSM 510 software.
Discussion
The ability of HIV-1 to exploit the immune responses that contribute to host defense is critical in the pathogenesis of AIDS.39 Because dendritic cells play an important role in the induction of immune responses, the modulation of their functional activity represents a strategic mechanism for HIV-1 to evade immune surveillance. HIV-1 envelope has been shown to modulate functions of various immune cells by binding to chemokine receptors CXCR4 and CCR5. The first cellular targets of HIV-1 infection during sexual transmission are immature dendritic cells present in the mucosal surface. These cells predominantly express CCR5, enabling chemotaxis in response to its cognate ligands, RANTES, MIP-1α, and MIP-1β.7 M-tropic HIV gp120 has also been shown to induce chemotaxis of iDCs, by binding to CCR5.10 However, very little is known about the pathways by which CCR5 signals in iDCs in response to either gp120 or their natural chemokine ligands. The present study indicates the central and novel role of the protein tyrosine kinase Pyk2-dependent pathway in HIV-1 envelope–induced CCR5-mediated chemotactic signaling in iDCs leading to its chemotaxis. This pathway is likely to play a critical role in HIV pathogenesis, by enhancing the trafficking and dissemination of HIV-1–infected DCs within the host.
In this study, we demonstrated that HIV-1 gp120 induces phosphorylation of Pyk2 at tyrosine residues 402 and 580. Tyr 402 has been shown to be the Pyk2 autophosphorylation site and binds to the SH2 domain of Src-family protein tyrosine kinases (SFKs).11 Binding of SFK allows for the subsequent phosphorylation of other tyrosine residues on Pyk2, leading to increased activity of Pyk2 and recruitment of additional SH2 domain–containing proteins.11 Tyr 579/580 is in the activation loop of the kinase domain and their phosphorylation correlates with increased Pyk2 kinase activity. Inhibition of Pyk2 activity by treatment with a tyrphostin A9, transduction with rAAV5 vector expressing a kinase-inactive mutant, as well as transfection of specific siRNA led to significant inhibition of M-tropic gp120–mediated migration. These results suggest that tyrosine phosphorylation and activation of Pyk2 plays an important role in HIV-1 gp120-induced migration of iDCs. Previously, Pyk2 has been shown to play a key role in CCR5-mediated signaling in macrophages and T cells.14,16,28,40 The first evidence of activation of Pyk2 in response to M-tropic gp120 was shown in primary CD4+ T cells.15 Del Corno et al later demonstrated that R5-gp120 activates a signaling cascade in macrophages that includes coreceptor binding and activation of Pyk2 and MAPK pathways leading to secretion of inflammatory mediators.16 With specific regard to dendritic cells, a recent study has shown that Pyk2 is involved in regulation of CCR7-mediated migratory speed of DCs in response to CCL19 and CCL21.41
In our study, we observed significant overexpression of the Pyk2 mutant using AAV vectors in dendritic cells. Several recent studies demonstrate that recombinant AAV vectors can be used to transduce DCs efficiently, mediating long-term gene expression and causing minimal toxicity.42-44 In addition, we achieved higher efficiency of transduction using AAV-5 vector in comparison with AAV-2. This is in agreement with a recent study that has shown a strong tropism of AAV5 serotype for human DCs.45 The high efficacy of AAV-5–based vectors in overexpressing Pyk2 could open up new possibilities for the development of novel AAV-vector–based vaccines, particularly, because the natural immunity to AAV5 is rare.45
Immature DCs may be exposed to free envelope protein as well as envelope on the surface of noninfectious virions in tissues. In this regard, we demonstrated that not only soluble gp120, but also virion-associated gp120, caused activation of Pyk2 leading to chemotaxis. AT-2–inactivated virus has been previously shown to modulate other DC functions such as maturation and cytokine production.46,47 Because, we used AT-2–inactivated HIV-viral preparations at a concentration that corresponds to 2 nM gp120 concentration, the particulate and trimeric virion-bound gp120 induced iDC chemotaxis comparable with soluble gp120. However, we found that a higher concentration of tyrphostin A9 (5 μM) was required to inhibit AT-2–inactivated HIV-1–induced iDC migration, in comparison with recombinant gp120-induced iDC migration. This difference is likely because the HIV-1 envelope is present as a multimeric form in AT-2–inactivated virus.
We also investigated the downstream components of the Pyk2-mediated pathway that regulates gp120-induced chemotaxis of iDCs. Our data indicate that gp120 induces activation of p38 MAP kinase, downstream of Pyk2. We observed significant inhibition of HIV gp120-induced migration in the presence p38 MAPK inhibitors (SB203580 and SB220025) compared with ERK inhibitor (PD98059). These results suggest that p38MAPK is a critical component of the HIV-1 gp120-induced chemotactic signaling pathway. A recent study has shown that exposure of iDCs to different R5–HIV-1 preparations leads to up-regulation of CCR7 and increased p38 MAPK phosphorylation.48 Furthermore, in this study, p38 MAP kinase activation was shown to regulate MIP-3β and SLC-induced migration of these cells.
Our studies also revealed that p38 MAP kinase phosphorylates serine residues of LSP1. LSP1 was in turn shown to associate with actin in the presence of gp120. Our findings are in agreement with an earlier finding in formyl-methionyl-leucyl-phenylalanine–treated neutrophils,35 where p38 MAP kinase has been shown to be an upstream regulator of LSP1. Confocal microscopic analysis revealed that M-tropic gp120 as well as AT-2–inactivated virus treatment induces redistribution of LSP-1 to cytoplasmic projections (uropod), and also its association with activated actin was predominantly observed at these sites. As far as dendritic cells are concerned, there is only one previous study that describes the role of LSP1 in facilitating the trafficking of HIV-1 into the proteasomes of DCs by interacting with DC-SIGN.49 In this study, it was also shown that down-regulation of LSP1 with specific siRNAs in human DCs enhanced HIV-1 transfer to T cells. In addition, bone marrow DCs from lsp1−/− mice showed an increase in transfer of HIV-1 (BaL) to a human T-cell line. However, to our knowledge, our study is the first report suggesting that LSP1 modulates cell motility in human iDCs.
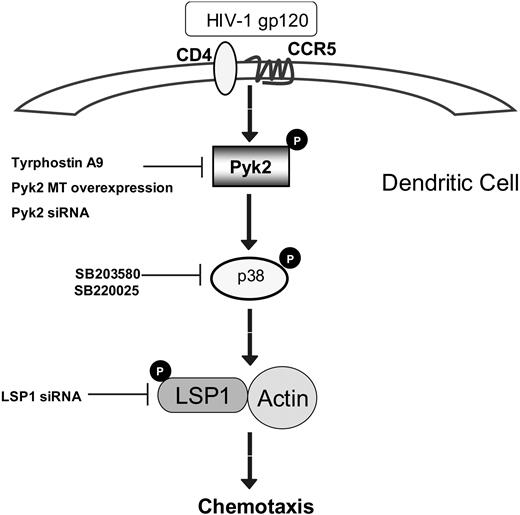
In summary, our studies demonstrate a novel signaling pathway regulating M-tropic HIV-induced chemotaxis of iDCs. We propose a model (Figure 7) whereby, as a result of interaction of gp120 with CCR5 on DCs, a signaling cascade is initiated that involves phosphorylation of the protein tyrosine kinase Pyk2, which in turn activates p38 MAP kinase. p38 MAP kinase activates LSP1, which then associates with actin, leading to consequent induction of chemotaxis. Thus, our studies reveal new insights into specific gp120-mediated DC activation pathways that may lead to DC dysfunction and contribute to the dissemination of the virus in the human body. Targeting novel signaling components such as Pyk2 and LSP1 may lead to innovative strategies in the treatment of AIDS.
Proposed scheme of the signaling pathway leading to immature dendritic cell chemotaxis upon exposure to M-tropic gp120.
Proposed scheme of the signaling pathway leading to immature dendritic cell chemotaxis upon exposure to M-tropic gp120.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by National Institutes of Health grant HL087576 (R.K.G.) and a grant from Ohio State University (R.K.G.).
National Institutes of Health
Authorship
Contribution: A.R.A. designed and performed research, analyzed data, and prepared the paper; A.P., R.R.B., Y.S.D., and T.N. designed and performed research and analyzed data; X.R. and E.F.T. contributed vital new reagents; and R.K.G. designed the research, analyzed data, and prepared the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ramesh K. Ganju, Department of Pathology, Ohio State University Medical Center, 1645 Neil Ave, 185 Hamilton Hall, Columbus, OH 43210; e-mail: ramesh.ganju@osumc.edu.
References
Author notes
A.R.A., A.P., and R.R.B. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal