Interleukin-33 (IL-33), a member of the IL-1 cytokine family, is emerging as a new regulator of immune responses and inflammatory vascular diseases. Although IL-33 and its cognate receptor ST2 appear to be expressed in vascular cells, the precise role of IL-33 in the vasculature has not been determined. In this study, we report a novel role of IL-33 as a potent endothelial activator, promoting both angiogenesis and vascular permeability. IL-33 increased proliferation, migration, and morphologic differentiation of human endothelial cells, consistently with increased angiogenesis in vivo. IL-33 also increased endothelial permeability with reduced vascular endothelial–cadherin-facilitated cell–cell junctions in vitro and induced vascular leakage in mouse skin. These effects of IL-33 were blocked by knockdown of ST2. Ligation of IL-33 with ST2 rapidly increased endothelial nitric oxide (NO) production through TRAF6-mediated activation of phosphoinoside-3-kinase, Akt, and endothelial NO synthase. Moreover, pharmacologic or genetic blockage of endothelial NO generation resulted in the inhibition of angiogenesis and vascular hyperpermeability induced by IL-33. These data demonstrate that IL-33 promotes angiogenesis and vascular leakage by stimulating endothelial NO production via the ST2/TRAF6-Akt-eNOS signaling pathway. These findings open new perspectives for the role of IL-33 in the pathogenesis of angiogenesis-dependent and inflammatory vascular diseases.
Introduction
Angiogenesis is a critical process in physiologic and pathologic conditions, including embryo development, wound healing, tumor progression, and inflammatory diseases.1 Inflammation and angiogenesis are closely associated, and pathologic angiogenesis has been implicated in the development of chronic inflammatory diseases. Interplay between inflammation and angiogenesis is mediated largely by cytokines, chemokines, and growth factors. Some of these molecules, including vascular endothelial growth factor (VEGF), induce endothelial permeability, allowing the infiltration of leukocytes to inflammatory sites, resulting in tissue damage.2
Interleukin-33 (IL-33) is a newly identified cytokine of the IL-1 family, which also includes the inflammatory cytokines IL-1α, IL-1β, and IL-18.3 It has been shown to signal via ST2 receptor.4 IL-33 expression is broadly detected in various tissues, including stomach, lung, spinal cord, brain, and skin, as well as in cells, including smooth muscle cells and epithelial cells lining bronchus and small airways.4 Notably, IL-33 expression is induced by IL-1β and tumor necrosis factor-α (TNF-α) in lung and dermal fibroblast and, to a lesser extent, by macrophage activation.4 IL-33 treatment has been shown to induce T-helper (Th) type 2 responses in mice as indicated by an increase in Th2 cytokine production and serum immunoglobulin. Systemic treatment of mice with IL-33 results in pathologic changes in the lung and the digestive tract. In the lung, vascular changes accompanied with eosinophilic and mononuclear infiltrates were observed in small muscular arteries.4 Recently, functions of IL-33 in cardiovascular diseases have been reported. For example, IL-33 can reduce the development of atherosclerosis in apolipoprotein E−/− mice on a high-fat diet.5 Furthermore, IL-33/ST2 complexes also have been shown to activate cardioprotective signaling pathways.6
IL-33 is produced as a 30-kDa precursor protein that is cleaved in vitro by caspase-1, releasing the mature 18-kDa form.4 Upon binding to the ST2 receptor, IL-33 promotes the activation of nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK), leading to increased transcription of Th2 cytokines.4 The ST2 receptor, a member of IL-1 receptor family, has long been known as an orphan receptor. Despite its structural and functional similarity to IL-1 receptor family, the ST2 receptor does not bind to IL-1α or IL-1β or other members of the IL-1 family. Two isoforms, a soluble (sST2) and a membrane bound form (ST2L), are produced through differential mRNA processing.7,8 ST2L is expressed mainly in mast cells and Th2 cells.7,8 Both ST2 forms also are expressed in freshly isolated human umbilical vein endothelial cells (HUVECs), and they are up-regulated by proinflammatory stimuli such as TNF, IL-1α, and IL-1β.9
Nitric oxide (NO) production in endothelial cells (ECs) is transiently regulated by multiple inflammatory angiogenic factors such as VEGF and angiopoietin-1.10,11 NO, in turn, modulates the angiogenic function of these factors. Recently, we demonstrated that receptor activator of NF-κB ligand (RANKL) promotes angiogenesis and vascular permeability through a NO-dependent mechanism.12 Endothelium-derived NO promotes angiogenesis and plays a critical role in both vascular remodeling and maintenance of vascular integrity.13 NO production in ECs relies on the endothelial NO synthase (eNOS) activity, which is regulated by various cytokines and environmental stimuli to modulate inflammation and angiogenesis.10,11 In eNOS mutant mice, postnatal angiogenesis in response to tissue ischemia is severely impaired, indicating that NO production has an essential role in postnatal neovascularization.10,14 Although the mechanisms by which NO regulates the angiogenic process are not fully understood, NO has emerged as an important modulator of physiologic and pathologic angiogenesis and inflammation.
Some of the cytokines implicated in inflammation can induce angiogenesis and increase vascular permeability and thus play a key role in regulating inflammatory angiogenesis. Therefore, the identification and characterization of angiogenic activities of cytokines are important for the development of therapeutics for vascular inflammatory diseases. Since the recent identification of IL-33 as a functional ligand of ST2, the immunomodulatory and inflammatory role of IL-33 have been well studied. However, the role of IL-33 in the vasculature has not been examined. We found that ST2 is highly expressed in ECs and thus hypothesized that IL-33 has a regulatory function in inflammatory angiogenesis. Here, we report for the first time that IL-33 has a significant effect on angiogenesis and vascular permeability through ST2 receptor binding. We further present genetic and pharmacologic evidence that endothelium-derived NO plays a critical role in promoting angiogenesis and vascular permeability induced by IL-33.
Methods
Cell culture and reagents
HUVECs were isolated from human umbilical cord veins by collagenase treatment, as described previously,15,16 and used at passages 2 to 7. The cells were grown in M199 medium (Invitrogen) supplemented with 20% fetal bovine serum (FBS), 100 units/mL penicillin, 100 μg/mL streptomycin, 3 ng/mL basic fibroblast growth factor (Upstate Biotechnology), and 5 units/mL heparin at 37°C under a humidified 95%/5% (vol/vol) mixture of air and CO2. Recombinant human IL-33 was purchased from Alexis Biochemicals. IL-33 diluted with M199 media was used in each experiment.
EC proliferation assay
HUVECs were seeded at a density of 2 × 104 cells per well in gelatin-coated 24-well plates. After 24 hours, they were washed twice with M199 and incubated for 5 hours in M199 containing 1% FBS. Cells were treated with various concentrations of IL-33 for 36 hours followed by the addition of 1 μCi/mL [3H] thymidine for 6 hours. High molecular weight DNA was precipitated with 10% trichloroacetic acid at 4°C for 30 minutes. Labeled DNA was solubilized in 0.2 N NaOH/0.1% sodium dodecyl sulfate (SDS) and counted by liquid scintillation counter (Perkin Elmer/Wallac).
EC migration assay
The chemotatic motility of HUVECs was assayed by the use of Transwell chambers (Corning Costar) with 6.5-mm diameter polycarbonate filters (8-μm pore size) as described previously.17 In brief, the lower surface of the filter was coated with 10 μg of gelatin. Fresh M199 medium (1% FBS) containing IL-33 was placed in the lower wells. The cells were trypsinized and suspended at a final concentration of 106 cells/mL in M199 containing 1% FBS. One hundred microliters of the cells' suspension were loaded into each of the upper wells, and the chamber was incubated at 37°C for 4 hours. The cells were fixed and stained with hematoxylin and eosin. Nonmigrating cells on the upper surface of the filter were removed by wiping them with a cotton swab, and chemotaxis was quantified with an optical microscope (×200) by counting cells that had migrated to the lower side of the filter.
Tube formation assay
Tube formation was assayed as previously described.12 In brief, 250 μL growth factor-reduced Matrigel (BD Biosciences) was pipetted into a 16-mm diameter tissue culture well and polymerized for 30 minutes at 37°C. HUVECs incubated in M199 containing 1% FBS for 6 hours were harvested after trypsin treatment, resuspended in M199, plated onto the layer of Matrigel at a density of 1.5 × 105 cells/well, and IL-33 was added. Matrigel cultures were incubated at 37°C. After 20 hours, the cultures were photographed (×200). The area covered by the tube network was determined with an optical imaging technique in which pictures of the tubes were scanned into Adobe Photoshop and quantified using ImageJ (National Institutes of Health).
[14C] sucrose permeability assay
HUVECs were plated onto a Transwell filter (Corning Costar). After reaching confluence, the cells were incubated with M199 containing 1% FBS for 3 hours and treated with various concentrations of IL-33. Fifty microliters (0.8 μCi/mL) [14C] sucrose (Amersham Pharmacia) was added to the upper compartment. The amount of radioactivity that diffused into the lower compartment was determined after 30 minutes with a liquid scintillation counter (Perkin Elmer/Wallac).
Immunofluorescence microscopy
HUVECs were fixed in 3.7% formaldehyde for 10 minutes and permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS). Cells were labeled with antivascular endothelial (anti-VE)–cadherin antibody (Santa Cruz Biotechnology) for 2 hours at room temperature, rinsed in PBS, and incubated with a fluorescein isothiocyanate–conjugated secondary antibody (Sigma-Aldrich) for 60 minutes at room temperature. Samples were examined with a fluorescence microscope (Zeiss; ×400).
Immunoprecipitation and Western blot analysis
HUVECs were lysed in 1 mL lysis buffer (20 mmol/L Tris/HCl, pH 8.0; 2 mmol/L ethylene diamine tetraacetic acid; 137 mmol/L NaCl; 1 mmol/L NaVO4; 1 mmol/L phenylmethylsulfonyl fluoride; 10% glycerol; and 1% Triton X-100). Lysates were clarified by centrifugation at 15 000g for 10 minutes, and the resulting supernatant were immunoprecipitated with antiphosphotyrosine (PY-20) antibody (BD Biosciences) at 4°C overnight, followed by the addition of protein A-agarose beads (Upstate Biotechnology) at 4°C for 1 hour. Immunoprecipitates were washed 3 times with lysis buffer, resuspended in SDS-polyacrylamide gel electrophoresis sample buffer containing β-mercaptoethanol, and further analyzed by Western blotting. For Western blot analysis, phosphorylated eNOS, phosphorylated Akt (P-Akt), eNOS, and Akt were purchased from Cell Signaling Technology.
Aortic ring assay
Aortas were harvested from 7-week-old C57BL/6 wild-type (WT) mice and sectioned into several pieces (aortic rings). Plates (48-well) were coated with 120 μL Matrigel. After gelling, the rings were placed in the wells. Recombinant mouse IL-33 protein was added to the wells in a final volume of 200 μL endothelial basal medium (EBM) (Lonza). As a control, aortic rings in EBM alone and VEGF-containing EBM were assayed. The plates were incubated at 37°C, and medium were changed every 2 days for 2 weeks. The angiogenic sprouting from aortic rings was examined in 5 rings per group (n = 5). Each aortic ring was photographed, and sprouting was quantified by counting the number of vascular sprouts that directly originated from mouse aorta. Photographed image was divided into 5 regions and spouts in each region were scored from 0 (least positive) to 1 (most positive) in a double-blind manner. The scores from 5 regions were combined, and total was scored from 0 (least positive) to 5 (most positive). Each data point was assayed in quintuple. All animal experiments were carried out with the approval of Yonsei University's Institutional Animal Care and Use Committee.
In vivo Matrigel plug assay
The Matrigel plug assay was performed as previously described.18 In brief, 7-week-old C57BL/6 mice (Orient Co) were injected subcutaneously with 0.6 mL of Matrigel containing the indicated amount of IL-33 and 10 units of heparin. The injected Matrigel rapidly formed a single, solid gel plug. After 6 days, the skin of the mouse was pulled back to expose the Matrigel plug, which remained intact. Hemoglobin was measured by the Drabkin method with Drabkin reagent kit 525 (Sigma-Aldrich) to quantify blood vessel formation. The concentration of hemoglobin was calculated by comparing to a known amount of hemoglobin assayed in parallel. To identify infiltrating ECs, immunohistochemistry was performed with anti-CD-31 antibody (BD Biosciences).
Miles vascular permeability assay
The Miles assay was performed as described previously.19 Evans blue dye (100 μL of a 1% solution in 0.9% NaCl) was injected into the tail vein of C57BL/6 WT and eNOS knockout (KO) mice. After 10 minutes, IL-33 (500 ng in 50 μL of PBS) was injected intradermally into the shaved back skin of mice. After 20 minutes, the animals were killed, and an area of skin that included the blue spot resulting from leakage of the dye was removed. Evans blue dye was extracted from the skin by incubation with formamide for 4 days at room temperature, and the absorbance of the extracted dye was measured at 620 nm with a spectrophotometer.
Intracellular NO detection
HUVECs were treated with 20 ng/mL IL-33 for 4 hours and then incubated with 5 μmol/L of 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate (Molecular Probes Inc) for 1 hour at 37°C. After the excess probe was removed, cells were incubated for an additional 20 minutes to allow for complete deesterification of the intracellular DAF-FM diacetate to the nonpermeable and nonfluorescent DAF-FM, which is converted to the highly fluorescent triazol form in the presence of NO. The fluorescence images were captured from at least 10 randomly selected cells per dish by the use of an Olympus IX81 microscope (Olympus). The relative levels of intracellular NO were determined from the fluorescence intensity of DAF-FM.
Transfection of siRNA
ECs were transfected with control siRNA (40 nmol/L), ST2 siRNA (40 nmol/L), and TRAF6 siRNA (40 nmol/L) by the use of lipofectamine (Invitrogen) for 3 hours. Cells were used for assays at 40 hours after transfection. Expression level of TRAF6 (Santa Cruz Biotechnology) and ST2 (R&D Systems) were determined by Western blotting. All small interfering (si)RNAs were designed by Dharmacon Inc. For TRAF6, the On-Target Plus SMARTpool (L-004712-00-0005) was used. For ST2, 2 different siRNAs with the sequence CGAAAGAGCAGGCGGCACAUUS or CCAGAAAGGCCUCUAGUUUUU were used.
Statistical analysis
Data are presented as mean plus or minus SD. Statistical comparisons between groups were performed by the use of one-way analysis of variance followed by the Student t test.
Results
IL-33 induces migration and capillary-like network formation of ECs
To examine the angiogenic activity of IL-33 in vitro, the effect of IL-33 on EC proliferation and migration was first evaluated by a thymidine incorporation assay and a modified Boyden Chamber Assay, respectively. HUVECs were treated with varying concentrations of recombinant human IL-33. IL-33 stimulated the proliferation as well as chemotactic motility of HUVECs with a maximal effect at 20 ng/mL (Figure 1A). Consistently, IL-33 increased the distances migrated from the wounded margin of HUVECs without significant effects on cell adhesion (supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article). The effect of IL-33 on morphologic differentiation of ECs was then investigated by the use of a 2-dimensional Matrigel assay. When plated on growth factor-reduced Matrigel in the absence of angiogenic factors, HUVECs formed incomplete and poorly connected tube networks. In contrast, treatment of IL-33 led to extensive tube networks, and more cell connections, compared with control-treated cells, within the tube networks were observed (Figure 1B; supplemental Figure 1C).
IL-33 induces migration, tube formation, and permeability of ECs. (A) Proliferative indices of HUVECs treated with various concentrations of IL-33 were accessed by [3H]thymidine incorporation assay. (B) Chemotactic motility of HUVECs induced by various concentrations of IL-33. HUVECs were placed in the upper chamber, and M199 (1% FBS) with various concentrations of IL-33 was placed in the lower wells of chemotaxis chamber. After 4 hours of incubation, chemotaxis was quantified by counting the cells that migrated to the lower side of the filter by optical microscopy at ×200 magnification. (C) HUVECs were plated on Matrigel-coated wells at a density of 1.5 × 105 cells/well with various concentrations of IL-33. After 20 hours, microphotographs were taken (×200). Capillary-like networks were quantified with ImageJ software. (D) HUVECs were incubated with various concentrations of IL-33 for 1 hour. Three independent experiments were performed in duplicate. (E) In vivo Miles vascular permeability assay. IL-33 or PBS was injected intradermally into the skin of C57BL/6 mice after intravenous injection of Evans blue. Data are means ± SDs; *P < .05, **P < .01 vs untreated control. (F) Confluent HUVECs were stained for VE-cadherin after cells were treated with IL-33 or VEGF for 1 hour. (G) VE-cadherin phosphorylation was assayed. HUVECs were grown to confluence and treated with 20 ng/mL IL-33 for the indicated time. Immunoprecipitated phosphotyrosine proteins were analyzed by SDS–polyacrylamide gel electrophoresis followed by immunoblotting with antibody to VE-cadherin.
IL-33 induces migration, tube formation, and permeability of ECs. (A) Proliferative indices of HUVECs treated with various concentrations of IL-33 were accessed by [3H]thymidine incorporation assay. (B) Chemotactic motility of HUVECs induced by various concentrations of IL-33. HUVECs were placed in the upper chamber, and M199 (1% FBS) with various concentrations of IL-33 was placed in the lower wells of chemotaxis chamber. After 4 hours of incubation, chemotaxis was quantified by counting the cells that migrated to the lower side of the filter by optical microscopy at ×200 magnification. (C) HUVECs were plated on Matrigel-coated wells at a density of 1.5 × 105 cells/well with various concentrations of IL-33. After 20 hours, microphotographs were taken (×200). Capillary-like networks were quantified with ImageJ software. (D) HUVECs were incubated with various concentrations of IL-33 for 1 hour. Three independent experiments were performed in duplicate. (E) In vivo Miles vascular permeability assay. IL-33 or PBS was injected intradermally into the skin of C57BL/6 mice after intravenous injection of Evans blue. Data are means ± SDs; *P < .05, **P < .01 vs untreated control. (F) Confluent HUVECs were stained for VE-cadherin after cells were treated with IL-33 or VEGF for 1 hour. (G) VE-cadherin phosphorylation was assayed. HUVECs were grown to confluence and treated with 20 ng/mL IL-33 for the indicated time. Immunoprecipitated phosphotyrosine proteins were analyzed by SDS–polyacrylamide gel electrophoresis followed by immunoblotting with antibody to VE-cadherin.
IL-33 induces endothelial cell permeability in vitro and in vivo
Because IL-33 has been implicated in various inflammatory responses and endothelial permeability is important in inflammation, we examined the effect of IL-33 on vascular permeability. IL-33 increased [14C] sucrose diffusion through the pores of Transwell membranes covered by HUVEC monolayer culture (Figure 1C). The maximal effect was observed at 20 ng/mL of IL-33, which is consistent with the EC migration response. Furthermore, the Miles assay showed that IL-33 treatment promoted vascular hyperpermeability in the mice skin (Figure 1E). EC permeability is regulated by junctional proteins, including VE-cadherin and occludin.20 We examined the effect of IL-33 on adherens junction formation by VE-cadherin by immunostaining ECs with anti–VE-cadherin. IL-33 reduced the localization of VE-cadherin at the cell–cell junction (Figure 1F; supplemental Figure 2). VE-cadherin phosphorylation is known to correlate with the loosening of adherens junctions at the cell–cell contacts of ECs, which is associated with transendothelial permeability. VEGF induces tyrosine phosphorylation of VE-cadherin, and this finding is concomitant with the increased vascular permeability.20,21 IL-33 increased tyrosine phosphorylation of VE-cadherin in HUVECs in a biphasic manner, with early and late responses (Figure 1G). The second wave of VE-cadherin phosphorylation is not likely caused by the secondary effect of VEGF induction because the blocking of VEGFR2 with neutralizing antibody did not significantly inhibit the phosphorylation of VE-cadherin at later time (supplemental Figure 3). Such biphasic behavior is frequently found in chemokine-stimulated processes.22,23 The ST2 receptors upon binding IL-33 can be internalized and recycled back to the surface at later times, potentially rebinding IL-33 and initiating new signaling events. These data suggest that IL-33 may affect vascular permeability in part by decreasing the stability of endothelial junctional complexes.
IL-33 induces angiogenesis ex vivo and in vivo
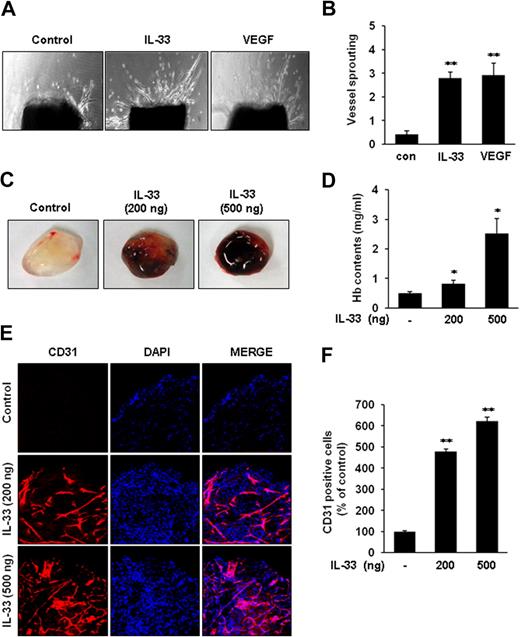
We next investigated the angiogenic activity of IL-33 in both ex vivo and in vivo angiogenesis models. First, the sprouting of vessels from mouse aortic rings was assessed to determine whether IL-33 induces angiogenesis ex vivo. Treatment with IL-33 resulted in approximately a 3-fold increase in vessel sprouting at mouse aortic rings compared with controls (Figure 2A-B). To further evaluate the angiogenic activity of IL-33 in vivo, we performed a mouse Matrigel plug assay. The Matrigel plugs containing IL-33 were red in color as the result of neovascularization (Figure 2C). Consistent with this observation, quantification of hemoglobin in the plugs revealed that the IL-33–treated Matrigel plugs contained more hemoglobin than control plugs (Figure 2D). The Matrigel plugs were immunohistochemically stained with anti-CD31 antibody for vessel density analysis. The CD31 staining also showed greater density of functional vasculature in the IL-33–treated plugs than in control plugs (Figure 2E-F). Taken together, these results demonstrate that IL-33 can induce angiogenesis in vivo as well as ex vivo.
IL-33 induces vessel sprouting ex vivo and angiogenesis in vivo. (A) Aortic segments were harvested from C57BL/6 mice. Aortic segments in Matrigel were treated with IL-33 (100 ng/mL) or VEGF (50 ng/mL) for 2 weeks (n = 5 per group). (B) Sprouting were classified from 0 (least positive) to 5 (most positive) as described in “Aortic ring assay.” (C-F) C57BL/6 mice were injected with 0.6 mL of Matrigel containing IL-33 (n = 6 per group). After 6 days, the mice were killed, and the Matrigel plugs were excised. (C) Representative Matrigel plugs were photographed. (D) Quantification of neovessel formation by measuring hemoglobin in the Matrigel. (E) Plugs were stained for infiltrating ECs by the use of anti-CD31 antibody. (F) Quantitative assessment of CD31+ ECs. Data are means ± SDs; *P < .05, **P < .01 vs untreated control.
IL-33 induces vessel sprouting ex vivo and angiogenesis in vivo. (A) Aortic segments were harvested from C57BL/6 mice. Aortic segments in Matrigel were treated with IL-33 (100 ng/mL) or VEGF (50 ng/mL) for 2 weeks (n = 5 per group). (B) Sprouting were classified from 0 (least positive) to 5 (most positive) as described in “Aortic ring assay.” (C-F) C57BL/6 mice were injected with 0.6 mL of Matrigel containing IL-33 (n = 6 per group). After 6 days, the mice were killed, and the Matrigel plugs were excised. (C) Representative Matrigel plugs were photographed. (D) Quantification of neovessel formation by measuring hemoglobin in the Matrigel. (E) Plugs were stained for infiltrating ECs by the use of anti-CD31 antibody. (F) Quantitative assessment of CD31+ ECs. Data are means ± SDs; *P < .05, **P < .01 vs untreated control.
ST2 is required for IL-33–induced angiogenesis and endothelial permeability
IL-33 has recently been identified as a functional ligand to the previously orphaned IL-1 family receptor ST2.4 The IL-33/ST2 complex was shown to have a regulatory role in the immune and inflammatory responses.4 Interestingly, we and others have detected the high expression of ST2 in ECs. Therefore, we determined the role of ST2 in the angiogenic activity of IL-33 by using siRNAs specific for ST2 (Figure 3A). The chemotatic motility induced by IL-33 was blocked in ST2 siRNA-treated ECs compared with control siRNA-treated cells (Figure 3B). Moreover, IL-33–induced, tube-like structure formation of ECs on Matrigel was impaired in ST2 siRNA-treated ECs (Figure 3C; supplemental Figure 4A). We further investigated the role of ST2 on endothelial permeability. Knockdown of ST2 also blocked the increase of endothelial permeability induced by IL-33 (Figure 3D). These data demonstrate that ST2 is an endothelial membrane receptor responsible for triggering angiogenesis and vascular permeability induced by IL-33.
IL-33–induced angiogenesis and vascular hyperpermeability is mediated by the ST2 receptor. (A-C) HUVECs were pretransfected with control siRNA (Con siRNA) or ST2 siRNA before IL-33 treatment. Cells were harvested for assay at 40 hours after transfection. (A) Expression levels of ST2 were determined by reverse-transcription polymerase chain reaction and Western blotting. (B) After IL-33 stimulation, chemotaxis was performed. (C) Cells were collected and replated on Matrigel-coated plates at a density of 1.5 × 105 cells/well and incubated with 20 ng/mL IL-33. Microphotographs were taken after 20 hours (×200). Tube networks were quantified with ImageJ software. (D) HUVECs were stimulated with IL-33 (20 ng/mL) for 1 hour. A [14C]sucrose permeability assay was then performed. Three independent experiments were performed in duplicate. Data are means ± SDs; *P < .05 vs control cell without IL-33; #P < .05 vs control cell with IL-33.
IL-33–induced angiogenesis and vascular hyperpermeability is mediated by the ST2 receptor. (A-C) HUVECs were pretransfected with control siRNA (Con siRNA) or ST2 siRNA before IL-33 treatment. Cells were harvested for assay at 40 hours after transfection. (A) Expression levels of ST2 were determined by reverse-transcription polymerase chain reaction and Western blotting. (B) After IL-33 stimulation, chemotaxis was performed. (C) Cells were collected and replated on Matrigel-coated plates at a density of 1.5 × 105 cells/well and incubated with 20 ng/mL IL-33. Microphotographs were taken after 20 hours (×200). Tube networks were quantified with ImageJ software. (D) HUVECs were stimulated with IL-33 (20 ng/mL) for 1 hour. A [14C]sucrose permeability assay was then performed. Three independent experiments were performed in duplicate. Data are means ± SDs; *P < .05 vs control cell without IL-33; #P < .05 vs control cell with IL-33.
IL-33 induces Akt/eNOS activation and NO production via ST2/TRAF6
The signaling of IL-33 via ST2 has been recently shown to initiate formation of a complex containing MyD88 and TNF-receptor associated factor (TRAF) 6.24 Importantly, we have recently found that the angiogenic effect of RANKL is mediated by a TRAF6-mediated NO pathway in ECs.25 Therefore, the possibility is raised that IL-33 may induce endothelial activation via TRAF6-mediated eNOS pathway. Several angiogenic molecules such as VEGF and RANKL are shown to signal through phosphoinoside-3-kinase (PI3K)/Akt to activate eNOS, which is an isoform of NO synthase constitutively expressed in ECs.26 eNOS is activated by Akt-mediated phosphorylation of Ser1177.27 We therefore tested whether IL-33 induces Akt/eNOS activation to produce NO in ECs. Western blot analysis showed that IL-33 increased the phosphorylation of Akt and eNOS, with a maximal effect at 20 ng/mL (Figure 4A). Phosphorylation of Akt/eNOS induced by IL-33 began at 10 minutes and was sustained for 60 minutes after treatment (Figure 4B). We further investigated the requirement of PI3K for the activation of Akt/eNOS with a PI3K-specific inhibitor, wortmannin. In contrast to the Src kinase inhibitor, PP1, wortmannin treatment suppressed the IL-33–induced phosphorylation of Akt/eNOS, indicating that PI3K is necessary for the activation of Akt/eNOS induced by IL-33 (Figure 4C). IL-33–induced NO production also was significantly blocked by the addition of wortmannin or NMA, a NO synthase inhibitor (Figure 4D). Moreover, the reduction of ST2 or TRAF6 expression with siRNAs inhibited the activation of Akt/eNOS and NO production (Figure 5A-D; supplemental Figure 4B), whereas overexpression of ST2 increased the phosphorylation of Akt/eNOS (supplemental Figure 5). These results indicate that ST2 and TRAF6 lie upstream of Akt in IL-33–induced eNOS activation. Taken together, these results demonstrate that IL-33 stimulates the production of endothelial NO via a ST2/TRAF6-mediated PI3K/Akt/eNOS signaling pathway.
IL-33 stimulates NO production in ECs via Akt/eNOS signaling pathway in ECs. (A-C) Phosphorylation of Akt and eNOS by IL-33 were determined by Western blotting. (A) HUVECs were stimulated with various concentrations of IL-33 for 30 minutes. (B) HUVECs were stimulated with 20 ng/mL IL-33 for the indicated times. (C) HUVECs were pretreated with 5 μmol/L PP1 (P) or 100 nmol/L wortmannin (W) for 30 minutes and then stimulated with 20 ng/mL IL-33 for 30 minutes. Blots are representative of 3 independent experiments. Densitometric analyses are presented as the relative ratio of P-Akt to Akt and P-eNOS to eNOS. The relative ratio in untreated control is arbitrarily presented as 100 (bottom). (D) HUVECs were pretreated for 30 minutes with inhibitors and then treated with 20 ng/mL IL-33 for 4 hours. ECs were incubated with DAF-FM diacetate for 1 hour at 37°C, and fluorescence images were captured with microscope. The relative levels of intracellular NO were quantified with Metamorph software (Molecular Devices). Three independent experiments were performed in duplicate. Data are means ± SDs; **P < .01 vs untreated control.
IL-33 stimulates NO production in ECs via Akt/eNOS signaling pathway in ECs. (A-C) Phosphorylation of Akt and eNOS by IL-33 were determined by Western blotting. (A) HUVECs were stimulated with various concentrations of IL-33 for 30 minutes. (B) HUVECs were stimulated with 20 ng/mL IL-33 for the indicated times. (C) HUVECs were pretreated with 5 μmol/L PP1 (P) or 100 nmol/L wortmannin (W) for 30 minutes and then stimulated with 20 ng/mL IL-33 for 30 minutes. Blots are representative of 3 independent experiments. Densitometric analyses are presented as the relative ratio of P-Akt to Akt and P-eNOS to eNOS. The relative ratio in untreated control is arbitrarily presented as 100 (bottom). (D) HUVECs were pretreated for 30 minutes with inhibitors and then treated with 20 ng/mL IL-33 for 4 hours. ECs were incubated with DAF-FM diacetate for 1 hour at 37°C, and fluorescence images were captured with microscope. The relative levels of intracellular NO were quantified with Metamorph software (Molecular Devices). Three independent experiments were performed in duplicate. Data are means ± SDs; **P < .01 vs untreated control.
IL-33 induces Akt/eNOS activation and NO production via ST2/TRAF6. (A-D) HUVECs were pretransfected with ST2 siRNA (40 nmol/L) and TRAF6 siRNA (40 nmol/L) before IL-33 treatment. Cells were harvested for assay at 40 hours after transfection. (A-B) HUVECs were treated with 20 ng/mL IL-33 for 30 minutes. Cells were harvested and phosphorylation of Akt and eNOS by IL-33 were detected by Western blotting. Blots are representative of 3 independent experiments. Densitometric analyses are presented as the relative ratio of P-Akt to Akt and P-eNOS to eNOS. The relative ratio in untreated control is arbitrarily presented as 100 (bottom). (C-D) After transfection, HUVECs were treated with 20 ng/mL IL-33 for 4 hours and incubated with DAF-FM diacetate for 1 hour. The relative levels of intracellular NO were determined from the fluorescence intensity of DAF-FM. Three independent experiments were performed in duplicate. Data are means ± SDs; *P < .01 vs control cell without IL-33; #P < .01 vs control cell with IL-33.
IL-33 induces Akt/eNOS activation and NO production via ST2/TRAF6. (A-D) HUVECs were pretransfected with ST2 siRNA (40 nmol/L) and TRAF6 siRNA (40 nmol/L) before IL-33 treatment. Cells were harvested for assay at 40 hours after transfection. (A-B) HUVECs were treated with 20 ng/mL IL-33 for 30 minutes. Cells were harvested and phosphorylation of Akt and eNOS by IL-33 were detected by Western blotting. Blots are representative of 3 independent experiments. Densitometric analyses are presented as the relative ratio of P-Akt to Akt and P-eNOS to eNOS. The relative ratio in untreated control is arbitrarily presented as 100 (bottom). (C-D) After transfection, HUVECs were treated with 20 ng/mL IL-33 for 4 hours and incubated with DAF-FM diacetate for 1 hour. The relative levels of intracellular NO were determined from the fluorescence intensity of DAF-FM. Three independent experiments were performed in duplicate. Data are means ± SDs; *P < .01 vs control cell without IL-33; #P < .01 vs control cell with IL-33.
PI3K/Akt/eNOS-mediated NO production is required for angiogenesis and vascular hyperpermeability stimulated by IL-33
To assess the role of PI3K/Akt/eNOS-mediated NO production in IL-33–induced angiogenesis and vascular permeability, we treated HUVECs with wortmannin or NMA before IL-33 treatment and examined the chemotactic motility and tubular network formation of ECs. The blockade of NO production with NMA abolished the effect of IL-33 on endothelial chemotaxis (Figure 6A) and tube formation on 2D Matrigel (Figure 6B; supplemental Figure 4C). Pretreatment with wortmannin also prevented the induction of EC chemotaxis and tube formation by IL-33 (Figure 6A-B; supplemental Figure 4C). Moreover, NMA and significantly suppressed IL-33–induced endothelial permeability in vitro (Figure 6C). Furthermore, we found that the vascular hyperpermeability of mouse skin induced by IL-33 was substantially impaired in the eNOS KO mice compared with WT mice (Figure 6D). These results demonstrate that IL-33–induced angiogenesis and vascular hyperpermeability is dependent on PI3K/Akt/eNOS-mediated NO production.
Impairment of IL-33–induced angiogenesis and vascular hyperpermeability in eNOS-deficient mice. (A-C) HUVECs were preincubated for 30 minutes with or without 100 nmol/L wortmannin or 1 mmol/L NMA before stimulation with IL-33 (20 ng/mL). (A) After 4 hours of incubation, chemotaxis was quantified by counting the cells that had migrated to the lower side of the filter by optical microscopy at ×200 magnification. (B) Cells were collected and replated on Matrigel-coated plates at a concentration of 1.5 × 105 cells/well. Microphotographs were taken after 20 hours (×200). (C) HUVECs were preincubated for 30 minutes with or without NMA (1 mmol/L) and then treated with 20 ng/mL IL-33 for 1 hour. A [14C]sucrose permeability assay was then performed. Three independent experiments were performed in duplicate. Data are means ± SDs; *P < .05, **P < .01 vs IL-33 alone. (D) In vivo Miles vascular permeability assay was performed in WT and eNOS KO mice. IL-33 (500 ng) and PBS were injected intradermally into the skin of mice after intravenous injection of Evans blue. Data are means ± SDs; **P < .01 vs IL-33 in eNOS KO.
Impairment of IL-33–induced angiogenesis and vascular hyperpermeability in eNOS-deficient mice. (A-C) HUVECs were preincubated for 30 minutes with or without 100 nmol/L wortmannin or 1 mmol/L NMA before stimulation with IL-33 (20 ng/mL). (A) After 4 hours of incubation, chemotaxis was quantified by counting the cells that had migrated to the lower side of the filter by optical microscopy at ×200 magnification. (B) Cells were collected and replated on Matrigel-coated plates at a concentration of 1.5 × 105 cells/well. Microphotographs were taken after 20 hours (×200). (C) HUVECs were preincubated for 30 minutes with or without NMA (1 mmol/L) and then treated with 20 ng/mL IL-33 for 1 hour. A [14C]sucrose permeability assay was then performed. Three independent experiments were performed in duplicate. Data are means ± SDs; *P < .05, **P < .01 vs IL-33 alone. (D) In vivo Miles vascular permeability assay was performed in WT and eNOS KO mice. IL-33 (500 ng) and PBS were injected intradermally into the skin of mice after intravenous injection of Evans blue. Data are means ± SDs; **P < .01 vs IL-33 in eNOS KO.
Discussion
We have shown that IL-33, a novel activator of ECs, promotes angiogenesis and vascular permeability in vitro and in vivo. IL-33 exerts its action in ECs through its cognate receptor, ST2, in NO-dependent signaling mechanisms. Vascular inflammation is associated with multiple diseases such as atherosclerosis, rheumatoid arthritis, and various cancers; therefore, our findings highlight the possibility that IL-33 is involved in the development of inflammatory vascular diseases.
IL-33, via the ST2 receptor, induces various types of inflammatory responses, particularly Th2- and mast-cell–dependent inflammation. Schmitz et al4 have shown that injection of IL-33 in mice causes inflammatory response-associated pathologic changes to the lung. IL-33 has been reported to promote allergic airway inflammation and exacerbate antigen-induced arthritis by activating mast cells.28,29 Contrary to its proinflammatory effect on other organs, the authors of recent studies have shown that IL-33 exerts protective effects in the cardiovascular system. For example, IL-33 treatment of apolipoprotein E−/− mice was shown to alleviate the atherosclerosis, a chronic inflammatory disease of the vasculature.5 It is notable that little inflammatory pathogenesis was found in the lung of this mouse model, which differs from the results of Schmitz et al.4,5 In addition, IL-33 treatment reduced cardiomyocyte hypertrophy and fibrosis in WT mice but not in ST2−/− mice.6 However, in the same study, IL-33 caused focal pulmonary inflammation in treated mice.6 The discrepancy in the lung pathology among these studies may be caused by various factors, such as the kinds and dosages of the recombinant IL-33 protein used for mouse injection, the administration methods, and mouse strain background. The differences between these studies indicate that further study is necessary to safely develop IL-33 as a therapeutic agent for the treatment of cardiovascular diseases. Here, for the first time, we demonstrate that IL-33 directly activates ECs, resulting in promoting angiogenesis and hyperpermeability. These properties of IL-33 in the vasculature may provide valuable clues for predicting the pathologic role of IL-33 in inflammatory vascular diseases and being considered as a therapeutic target.
The early stages of angiogenesis often are accompanied by increased vascular permeability, although vascular leakage is not a prerequisite for vessel growth. Increased vascular permeability is also found in areas of diseased tissue associated with diabetic retinopathy, solid tumors, myocardial infarction, wounds, and chronic inflammation.30,31 Many angiogenic factors are known to regulate vascular permeability. VEGF, the best known angiogenic stimulus, was originally identified as a vasopermeability factor that increases the vascular permeability of microvessels to circulating macromolecules.32,33 The vasopermeability factor activity of VEGF is involved in numerous instances of pathologic angiogenesis.34 However, whereas VEGF promotes both angiogenesis and vascular permeability, other angiogenesis stimulatory factors such as FGF and platelet-derived growth factor have no significant effect on permeability.30 In the case of angiopoietin-1, it inhibits endothelial permeability despite its prominent angiogenic activity.35 IL-33, like VEGF, induces both angiogenesis and vasopermeability. IL-33 increased the EC proliferation, migration, and tubular network formation comparable with VEGF in vitro (Figure 1A-C). Consistently, IL-33 promoted vessel sprouting from explanted mouse aortic rings and neovascularization in Matrigel plugs inserted in mice skin (Figure 2). EC junctional permeability in vitro as well as vessel leakage in mice was also increased by IL-33 treatment (Figure 1D-E). Some cytokines that induce inflammatory angiogenesis, such as IL-1β, exert their action through up-regulation of VEGF.36,–38 However, the effect of IL-33 on the vasculature is not likely to be mediated by VEGF because IL-33 does not increase the expression of VEGF in ECs (supplemental Figure 6). Furthermore, blocking KDR (VEGFR2)–mediated VEGF signaling with neutralizing antibody did not significantly inhibit IL-33 activation of PI3K/Akt-eNOS signaling, suggesting that the angiogenic effect of IL-33 may not be the secondary effect of VEGF induction (supplemental Figure 6).
IL-33 is produced by several cell types and exists both as a secreted form and a nuclear form like IL-1α and the high-mobility-group B.4,39,40 The nuclear form of IL-33 has been reported to be a transcription regulator and bind to heterochromatin. Interestingly, nuclear IL-33 was first identified in specialized ECs present in high endothelial venules and abundantly detected in inflammatory regions. Although the reverse expression pattern of nuclear IL-33 to endothelial activation was reported,41 its precise role in endothelial activation has not been determined. Considering distinct roles of nuclear and secreted forms of other cytokines such as IL-1α, nuclear IL-33 may also work differently from secreted IL-33 on ECs. The full-length precursor IL-33 was shown to be cleaved by caspase-1 in vitro.4 However, it has not been determined whether IL-33 is a substrate of caspase-1 in vivo. Notably, caspase-1 is known as a cysteine protease that has importance as an inflammatory mediator, and its activity is increased under hypoxic and ischemic environments.42,43 Indeed, caspase-1 is shown to play a key role in inflammatory pathways by processing pro-IL-1β into mature IL-1β.43 Therefore, it is speculated that vascular injury, including ischemia, could induce the secretion of IL-33 from cells by activating caspase-1, and secreted IL-33 may contribute to inflammation in multiple ways, including endothelial activation as well as activation of mast cells or other immune cells. It is also noteworthy that the expression of IL-33 and its corresponding receptor, ST2, in ECs are further modulated by various inflammatory cytokines such as IL-1β and TNF-α.4,9
Our results reveal the prominent roles of endothelial NO in IL-33–induced angiogenesis and vascular permeability in vitro and in vivo. IL-33 stimulates NO production in ECs via PI3K/Akt-dependent eNOS activation, which is required both for angiogenesis and vascular permeability (Figure 4; Figure 6). Many, although not all, angiogenic factors such as VEGF and RANKL exert their angiogenic and vasopermeability activities in NO-dependent manners.12,14,25,44 NO generated by eNOS has been implicated in both physiologic and pathologic angiogenesis and inflammation. Indeed, eNOS mutant mice have altered vascular remodeling and impaired angiogenesis.45 Furthermore, these mice have shown impaired wound healing and postnatal angiogenesis in response to tissue ischemia.14 NO also appears to be important in regulating vascular permeability, although this function is context dependent. In hindlimb-ischemia models, NO has been shown to maintain vessel integrity.14 In contrast, it increases vascular permeability in tumors and in chronic inflammation.30 In this study, we observed that treatment of ECs with IL-33 leads to an increase in NO production, which was associated with increased eNOS activity (Figure 4). Blockade of NO release with a specific inhibitor or genetic means prevented IL-33–induced angiogenesis and vascular permeability (Figure 6A-C). Thus, it is clear that endothelium-derived NO is required for the angiogenic and permeability responses to IL-33. Interestingly, the use of NO and endothelial functions of IL-33 is quite similar to those of VEGF and RANKL, which are considered as an inflammatory and angiogenic factors in adult. Thus, these properties suggest that IL-33 may be involved in the pathogenesis of inflammatory vascular diseases.
Our study also provides new insight into the molecular mechanism of IL-33 function in promoting angiogenesis and vascular permeability. IL-33 signals via the ST2 receptor, which is a member of IL-1 and Toll-like receptor (TLR) superfamily. A recent study46 has demonstrated that IL-33 also requires the binding of IL-1R accessory protein (IL-1RAcP) for its signal transduction. Similar to other members of the IL-1 family of cytokine, the ST2/IL-1RAcP complex, when bound by IL-33, signals through TRAF6 and leads to the activation of various intracellular signaling pathways, including NF-κB and/or MAPK.4 Our present data revealed that IL-33 upon receptor ligation triggers PI3K/Akt-dependent eNOS signaling pathway in ECs. IL-33 increased phosphorylation of Akt and eNOS in ECs, and this effect was blocked by a PI3K-specific inhibitor (Figure 4). Importantly, PI3K/Akt has been shown to be involved in the TRAF6-mediated signaling pathway.47 For instance, TRAF6 activates PI3K/Akt pathway in ECs in response to lipopolysaccharide,47 and TRAF6 activation is required for lipopolysaccharide-mediated angiogenic response in vitro and in vivo.48 In addition, our previous study demonstrated that RANKL induces angiogenesis and vasopermeability through TRAF6-mediated PI3K/Akt/eNOS activation and consequent NO production.12 Most likely, our present data showed that IL-33 activates the PI3K/Akt/eNOS signaling cascade in a TRAF6-dependent manner (supplemental Figure 7). Therefore, it is suggested that TRAF6 could be an important mediator of endothelial activation in response to various inflammatory stimuli.
In summary, the present study provides the first evidence that IL-33 promotes angiogenesis and endothelial permeability in an endothelium-derived NO-dependent manner. These findings suggest that elevated IL-33 levels in the vascular area may lead directly to endothelial activation and may make an important contribution to the pathogenesis of angiogenesis-dependent inflammatory vascular diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a National Research Laboratory grant (20090083119); a grant from the 21C Frontier Functional Human Genome Project (2009K001055), Ministry of Education, Science, and Technology (MEST); a grant from the Korea Health 21 R&D Project, Ministry of Health Welfare & Family Affairs, Republic of Korea (A085136); and a Korean Research Foundation Grant funded by the Korean government (KRF-2006-312-C00611).
Authorship
Contribution: Y.-S.C., H.-J.C., and Y.-G.K. planned the experimental design, analyzed data, and wrote the paper; B.-J.P. and H.-R.P. performed the in vivo Matrigel plug experiments; Y.-S.M. performed the Miles assay; J.-K.M. and J.-H.K. performed the siRNA experiment; and Y.-M.K. provided NO knockout mice and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Young-Guen Kwon, Department of Biochemistry, College of Life Science and Biotechnology, Yonsei University, Seoul 120-749, Republic of Korea; e-mail: ygkwon@yonsei.ac.kr.

![Figure 1. IL-33 induces migration, tube formation, and permeability of ECs. (A) Proliferative indices of HUVECs treated with various concentrations of IL-33 were accessed by [3H]thymidine incorporation assay. (B) Chemotactic motility of HUVECs induced by various concentrations of IL-33. HUVECs were placed in the upper chamber, and M199 (1% FBS) with various concentrations of IL-33 was placed in the lower wells of chemotaxis chamber. After 4 hours of incubation, chemotaxis was quantified by counting the cells that migrated to the lower side of the filter by optical microscopy at ×200 magnification. (C) HUVECs were plated on Matrigel-coated wells at a density of 1.5 × 105 cells/well with various concentrations of IL-33. After 20 hours, microphotographs were taken (×200). Capillary-like networks were quantified with ImageJ software. (D) HUVECs were incubated with various concentrations of IL-33 for 1 hour. Three independent experiments were performed in duplicate. (E) In vivo Miles vascular permeability assay. IL-33 or PBS was injected intradermally into the skin of C57BL/6 mice after intravenous injection of Evans blue. Data are means ± SDs; *P < .05, **P < .01 vs untreated control. (F) Confluent HUVECs were stained for VE-cadherin after cells were treated with IL-33 or VEGF for 1 hour. (G) VE-cadherin phosphorylation was assayed. HUVECs were grown to confluence and treated with 20 ng/mL IL-33 for the indicated time. Immunoprecipitated phosphotyrosine proteins were analyzed by SDS–polyacrylamide gel electrophoresis followed by immunoblotting with antibody to VE-cadherin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/14/10.1182_blood-2009-02-203372/5/m_zh89990942750001.jpeg?Expires=1765903313&Signature=mKf3LcQU2Ls-wktTG60AVbidYaM6nuiEwNXiI6OnphvBPRp0-MYYJODyqIDYfZCR399XhJnYGPeJ49brvRR6VBapfUGS3I4RPeZf3ezB5causQqmPJKll8mSyfwI8KdBZ~QdTeIRbE82PCBo5-1C0VEgmuuYouospkQMf~81KOwT--kwlBPMFSnR6vHJGRzvMiCEBExIKYfW5MSCjtIJAVngNjeviX7xEaVzejoNVi1m-0KEu1MigsmQVeXqeOzkBYnR5c2583Fc8U4zFehd9XmRb3cXQwOaeOfLCb-vdFqrrQ0nuyXMCpovtUnULGwZNkb7LMJ77H8jUGErD9dd9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. IL-33–induced angiogenesis and vascular hyperpermeability is mediated by the ST2 receptor. (A-C) HUVECs were pretransfected with control siRNA (Con siRNA) or ST2 siRNA before IL-33 treatment. Cells were harvested for assay at 40 hours after transfection. (A) Expression levels of ST2 were determined by reverse-transcription polymerase chain reaction and Western blotting. (B) After IL-33 stimulation, chemotaxis was performed. (C) Cells were collected and replated on Matrigel-coated plates at a density of 1.5 × 105 cells/well and incubated with 20 ng/mL IL-33. Microphotographs were taken after 20 hours (×200). Tube networks were quantified with ImageJ software. (D) HUVECs were stimulated with IL-33 (20 ng/mL) for 1 hour. A [14C]sucrose permeability assay was then performed. Three independent experiments were performed in duplicate. Data are means ± SDs; *P < .05 vs control cell without IL-33; #P < .05 vs control cell with IL-33.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/14/10.1182_blood-2009-02-203372/5/m_zh89990942750003.jpeg?Expires=1765903313&Signature=OFOMVE7~ha03LyFCzvinCc8EBOkSXzA55Q9ApEOUnB4YrglLCQ0rZvU~FAsqJP6CRAealhgONFQQxbdTIxy6JWGBxUOLiwBYP2EmkkkoUdIYRr6e~BTp3Sc1hcOhCKllpznxfBhLzgRrYJFn6CfNGxu8GQiBgOfxwioAdum67HSs~8bia1EVSRxpIyYvaS1hJGYUOaPBov5lJuLmGzKqEVPYLspNdMhMTHE3DNdIcTfAzwDC-QxXzqg2rJa9sMQlf77GIW3CdY-nnSU3~VDR1K~nljSotxvD1zScAEbTYmGU9lMXTVXVk8ed2Pt7x3SndD9uX4-tj5wO8RYoFbpP6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 6. Impairment of IL-33–induced angiogenesis and vascular hyperpermeability in eNOS-deficient mice. (A-C) HUVECs were preincubated for 30 minutes with or without 100 nmol/L wortmannin or 1 mmol/L NMA before stimulation with IL-33 (20 ng/mL). (A) After 4 hours of incubation, chemotaxis was quantified by counting the cells that had migrated to the lower side of the filter by optical microscopy at ×200 magnification. (B) Cells were collected and replated on Matrigel-coated plates at a concentration of 1.5 × 105 cells/well. Microphotographs were taken after 20 hours (×200). (C) HUVECs were preincubated for 30 minutes with or without NMA (1 mmol/L) and then treated with 20 ng/mL IL-33 for 1 hour. A [14C]sucrose permeability assay was then performed. Three independent experiments were performed in duplicate. Data are means ± SDs; *P < .05, **P < .01 vs IL-33 alone. (D) In vivo Miles vascular permeability assay was performed in WT and eNOS KO mice. IL-33 (500 ng) and PBS were injected intradermally into the skin of mice after intravenous injection of Evans blue. Data are means ± SDs; **P < .01 vs IL-33 in eNOS KO.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/14/10.1182_blood-2009-02-203372/5/m_zh89990942750006.jpeg?Expires=1765903314&Signature=XFyyQxOvSmEt67Vg~1sZXJ3SdBHyJ2cqh8Zyd370UJ7UAMlsmTGbX3p-gbazJDhYwUxgasMtcPq9W4Dgm5lzIrbj~dFxqCAVRRtPJChuZ4ov0LKwughEzjRJ8na8FHU2DZO2n8R-GfOQJzyYr74LQrMl3DvMNM08D8MQ6fACWUfz8SdtL4jm3-44dGkcqhhHreTrDETKjrYJNiMmAvWY-zEKt3oJeie1o1x67bwAeZCA65z08926XEXtGFto~auTfaxy-MBwUV~MmWyvRn4HTttocmNtSZD6UOKH~Eh0rE8DVon75pY6Ouv4dyF9BL3mowZfiPLeNNNF~ddW8Vt00Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal