Antiphospholipid (aPL) antibodies recognize receptor-bound β2 glycoprotein I (β2GPI) on target cells, and induce an intracellular signaling and a procoagulant/proinflammatory phenotype that leads to thrombosis. Evidence indicates that annexin A2 (A2), a receptor for tissue plasminogen activator and plasminogen, binds β2GPI on target cells. However, whether A2 mediates pathogenic effects of aPL antibodies in vivo is unknown. In this work, we studied the effects of human aPL antibodies in A2-deficient (A2−/−) mice. A2−/− and A2+/+ mice were injected with immunoglobulin G (IgG) isolated from either a patient with antiphospholipid syndrome (IgG-APS), a healthy control subject (IgG-normal human serum), a monoclonal anti-β2GPI antibody (4C5), an anti-A2 monoclonal antibody, or monoclonal antibody of irrelevant specificity as control. We found that, after IgG-APS or 4C5 injections and vascular injury, mean thrombus size was significantly smaller and tissue factor activity was significantly less in A2−/− mice compared with A2+/+ mice. The expression of vascular cell adhesion molecule-1 induced by IgG-APS or 4C5 in explanted A2−/− aorta was also significantly reduced compared with A2+/+ mice. Interestingly, anti-A2 monoclonal antibody significantly decreased aPL-induced expression of intercellular cell adhesion molecule-1, E-selectin, and tissue factor activity on cultured endothelial cells. Together, these data indicate for the first time that A2 mediates the pathogenic effects of aPL antibodies in vivo and in vitro APS.
Introduction
Antiphospholipid syndrome (APS) is a multisystem, autoimmune disorder characterized by recurrent thrombosis, pregnancy loss, and thrombocytopenia, in association with the presence of antiphospholipid (aPL) antibodies (Abs) and persistently positive anticardiolipin (aCL) and/or lupus anticoagulant tests.1,2 It is now well established that aPL Abs are heterogeneous and bind to various protein targets. Among these, the plasma protein β2 glycoprotein I (β2GPI) is the primary target antigen,3 and interacts with diverse cell types, receptors, and enzymes.4,,–7 β2GPI has been shown to bind to different types of endothelial cells (EC), the main tissue targets for thrombosis, as well as trophoblasts and decidual cells, the main targets for defective placentation and fetal loss.8,,–11
Studies that used animal models of thrombosis indicate that aPL Abs are pathogenic as they induce EC activation and pregnancy loss when given in vivo.12,–14 aPL Abs are believed to promote thrombosis in several ways. They appear to interfere with a range of normal cell surface hemostatic mechanisms by targeting coagulation factors, natural anticoagulants, oxidized low-density lipoproteins, CD36, and fibrinolytic proteins.4,,–7,15,,,–19 Emerging evidence suggests that plasma hypofibrinolysis is a risk factor for venous thrombosis,20 and that fibrinolysis might be impaired in APS due to increased fibrinolytic inhibitor (plasminogen activator inhibitor type-1) activity.21
Annexin A2 (A2) is a profibrinolytic receptor that binds both plasminogen and its activator, tissue plasminogen activator (tPA), functioning as a cofactor for plasmin generation, and localizing fibrinolytic activity to the EC surface. A2 is found on the surface membrane of ECs and monocytes, and also on the brush-border membrane of placental syncytiotrophoblasts, all of which are recognized targets of pathogenic aPL Abs.22,23 Several lines of evidence indicate that A2 acts as a tPA-dependent cofactor for cell surface plasmin generation in vivo.24,,–27
Investigators have shown that ECs express significantly higher amounts of adhesive glycoproteins, such as intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin (E-sel), when incubated with aPL Abs and β2GPI in vitro.8,28 Our group has shown that aPL Abs activate endothelium both in vitro and in vivo, and these observations correlate with enhancement of thrombus formation in the mouse.12,29,30 In addition to these proinflammatory effects, aPL Abs up-regulate tissue factor (TF) expression and function on monocytes and ECs.31,32 Additional studies have reported higher plasma levels of TF in APS patients than controls.33,34 Hence, there is convincing evidence that aPL Abs induce EC and monocyte activation, leading to a procoagulant and proinflammatory phenotype in vitro and in vivo.
Previous studies have shown that A2 mediates EC activation by aPL/anti-β2GPI Abs after binding to β2GPI.35,36 However, an understanding of how A2 mediates the pathogenic effects of aPL Abs in vivo is lacking. To investigate this question further, we examined the role of A2 in aPL pathogenicity in vitro and in vivo. We studied the effect of an anti-A2 Ab on aPL Ab-induced up-regulation of ICAM-1, E-sel, and TF on cultured ECs in vitro. We also examined the effect of aPL (polyclonal and monoclonal) Abs on in vivo thrombus formation, aortic VCAM-1 expression, and carotid artery TF function in A2−/− mice.
Methods
Preparation of immunoglobulin G
Total immunoglobulin G (IgG) containing aPL Abs (IgG-APS) from one patient with primary APS (without systemic lupus erythematosus) was affinity purified using protein G-Sepharose chromatography, as previously described.12 The patient was a 53-year-old white male with a history of 1 transient ischemic attack, 2 myocardial infarctions, 3 deep vein thromboses, and 1 pulmonary embolism. The titers of aCL and anti-β2GPI Abs in his serum were 456 G phospholipid units (GPL)/mL and 256 standard G units (SGU)/mL, respectively, and the lupus anticoagulant test was positive.37,38 The APS serum used in these experiments also had anti-A2 activity (optical density [O.D.] values of a 1/50 dilution were 0.734 U), determined by enzyme-linked immunosorbent assay (ELISA) using recombinant A2 (kindly provided by K. McCrae, Case Western Reserve University, Cleveland, OH), as described elsewhere.19 Human IgG from a healthy person (IgG–normal human serum [NHS]) was purified by an identical method. The protein concentration was determined by the Bradford method (Bio-Rad Quick Start Bradford Protein Assay).39 Volunteers who donated serum for this study signed a consent form approved by the Institutional Review Board committee of the University of Texas Medical Branch, in accordance with the Declaration of Helsinki.
A murine monoclonal Ab (named 4C5) with specificity for human and murine β2GPI was prepared, as described elsewhere.40 The 4C5 was shown to bind to cardiolipin in a β2GPI-dependent fashion, to have lupus anticoagulant and thrombogenic activity, and to activate ECs in previous studies.40 The purified IgG was dialyzed against Tris-buffered saline, and the concentration of the protein was determined by the Bradford method.39
A murine anti-A2 monoclonal Ab (anti-A2 MoAb) was used in some experiments (clone 5; BD Biosciences). A murine monoclonal of irrelevant specificity (MuMoAbC) was used as a control (mouse IgG1k MPOC-21; Sigma-Aldrich).
Western blotting to detect antigen specificity
Total proteins were obtained from confluent human umbilical vein ECs (HUVECs) and cardiac microvascular ECs from A2+/+ and A2−/− mice, as described.41 Purified human β2GPI protein was obtained from Haematologic Technologies. Binding specificity of mouse monoclonal IgG (4C5), used at 30 μg/mL; MuMoAbC, used at 30 μg/mL; mouse monoclonal anti-A2 IgG used at 0.25 μg/mL; and mouse monoclonal anti–glyceraldehyde-3-phosphate dehydrogenase IgG (Biodesign International), used at 1 μg/mL was assessed by Western blotting.42 The 4C5 and MuMoAbC were tested against the same molecular weight proteins.
In vitro experiments
Cell culture.
An established HUVEC cell line (ATCC) was propagated at 37°C in Kaighn's F12K medium (Invitrogen), supplemented with 0.1 mg/mL heparin, 0.03 to 0.05 mg/mL EC growth supplement, and 10% fetal bovine serum on collagen-coated tissue culture flasks. Cell cultures were maintained in a 5% CO2-humidified incubator. All experimental data were obtained using 2nd to 6th passage HUVECs at 1 to 2 days postconfluence.
In vitro detection of surface E-sel and ICAM-1 on EC.
To test the ability of IgG-APS to modulate the expression of adhesion molecules, E-sel and ICAM-1 were assessed by ELISA, as previously described.12 HUVECs were then treated for 4 hours at 37°C with normal IgG (IgG-NHS: 200 μg/mL in F12K medium), IgG-APS (200 μg/mL in F12K medium), IgG-APS plus mouse monoclonal anti-A2 IgG (clone 5; BD Biosciences; 1.0 μg/mL added), IgG-APS plus mouse control (MuMoAbC; Sigma-Aldrich), or medium alone. As a positive control, some monolayers were treated with lipopolysaccharide (LPS; 5 μg/mL; Santa Cruz Biotechnology). Later, HUVEC monolayers were fixed with 2% paraformaldehyde and then incubated with phosphate-buffered saline (PBS)/1% bovine serum albumin (1 hour, 21°C) to block nonspecific binding.12,29 The fixed monolayers were incubated in turn with anti–E-sel (Sigma-Aldrich) or ICAM-1 (Serotec; 1:1000 in blocking solution) and a peroxidase-conjugated goat anti–mouse IgG (Santa Cruz Biotechnology) for 1 hour. Tetramethylbenzidine solution (ScyTek Laboratories) was added to each well. The color reaction was stopped by addition of 8 N sulfuric acid. O.D. were read at 450 nm in an ELISA reader. In all experiments, a second-step background control was performed in which cells were treated, as described above, but without incubation with first Ab. The degree of specific antigen expression was calculated by subtracting nonspecific binding of the secondary Ab from all test values. Experiments were run in triplicate and performed 3 times.
TF functional assay.
EC TF activity was assessed, as previously described.32 Briefly, HUVECs were grown in 6-well tissue culture plates, as described above. Eighteen hours before each experiment, medium was replaced with F12K medium containing 1% fetal bovine serum. Cells were then treated (4 hours, 37°C) with normal IgG (IgG-NHS: 200 μg/mL in F12K medium), IgG-APS (200 μg/mL in F12K medium), IgG-APS plus mouse anti-A2 MoAb (0.5 μg/mL added), or medium alone. As a positive control, monolayers were treated with LPS (5 μg/mL; Santa Cruz Biotechnology). The cells were then washed with cold PBS and scraped from the dishes into 1 mL PBS. The preparations were centrifuged for 5 minutes at 2700g and resuspended in 100 μL PBS-0.1% Triton X-100 buffer. Cells were lysed by sonication, and lysates were stored at −20°C until used. TF activity was determined using a commercial chromogenic kit (Actichrome TF; American Diagnostica) that measures factor Xa after activation by TF-factor VII complex. The amount of factor Xa generated is measured by its ability to cleave Streptozyme Xa, a highly specific chromogenic substrate for factor Xa. The protein concentration from lysates was evaluated by Bradford.39 Final TF concentrations were expressed as TF pM (pmol)/protein concentration.32 Experiments were performed 3 times in duplicate.
In vivo experiments
Analysis of thrombus dynamics.
A2−/− mice weighing approximately 20 g were obtained from Dr Hajjar's laboratory and housed in the animal care facilities of the University of Texas Medical Branch (American Association of Laboratory Animal Care approved). Wild-type mice (C57BL/6J mice) were obtained from The Jackson Laboratory. Animals were handled by trained personnel according to Institutional Animal Care and Use Committee guidelines. Mice (5-10 animals/group) were injected intraperitoneally with 500 μg IgG-APS, IgG-NHS, 100 μg 4C5, 100 μg anti-A2 MoAb, or 100 μg MuMoAbC twice (at time 0 and 48 hours later). Surgical procedures were performed to study thrombus dynamics 72 hours after the first injection, as previously described.12,29,30 Briefly, the femoral vein was pinched using a standard pressure to introduce injury and induce a clot. Clot formation and dissolution in the transilluminated vein were visualized with a microscope equipped with closed-circuit video system. When a thrombus reached maximum size, its area was measured (in square microns [μm2]) by digitizing the image and tracing the outer margin of the thrombus. Two to 3 thrombi were induced in each animal, and the mean thrombus area was computed for each group of animals. In addition, as discussed below, TF activity in carotid artery samples and endothelial VCAM-1 expression were carried out in the mice after the analysis of thrombus dynamics. The aCL and anti-β2GPI titers in the sera of the mice were determined, as described previously.12,29,30
Determination of TF activity in carotid artery homogenates.
Pieces of approximately 5 mm of uninjured right and left carotid arteries were dissected and collected from each animal into Tris-buffered saline–0.1% Triton X-100 buffer containing heparin as anticoagulant, and homogenized, as described elsewhere.43,44 The TF activity in the carotid artery homogenates was then determined using a commercial chromogenic assay (Actichrome TF; American Diagnostica) that measures factor Xa after activation by the TF-factor VII complex. TF activity was expressed in pmol/mg/mL−1 protein.
Determination of endothelial VCAM-1 expression in en face preparations of mouse aorta using quantum dot bioconjugates and 2-photon excitation laser-scanning microscopy.
A2−/− mice (n = 2 per treatment group) or the corresponding wild-type animals were injected intraperitoneally with 500 μg/mL IgG-APS twice, 500 μg/mL IgG-NHS, 100 μg/mL 4C5, 100 μg/mL anti-A2 MoAb, or 100 μg/mL MuMoAbC, as described in the previous section. Other A2−/− and A2+/+ mice were challenged with LPS (1 μg/g body weight, intraperitoneally), or with PBS alone 4 hours before euthanasia. A comprehensive description of this methodology can be found elsewhere.45,,,,–50 In short, animals were euthanized by CO2 inhalation, and the aortas were pressure perfused at 100 mmHg with cold 0.9% NaCl solution, followed by pressure fixation in 10% formalin. After washing, the descending thoracic aortas were incubated overnight at 4°C with rat anti–mouse monoclonal Ab directed against VCAM-1 (1/50 dilution; BD Biosciences). Three washing steps were performed to remove primary Ab excess before the incubation for 1 hour at room temperature with quantum dot bioconjugates (Qdot 655 goat F(ab′)2) anti–rat IgG conjugate (Invitrogen). Finally, nuclear counterstaining was performed with Hoechst dye.
Image collection was accomplished using a custom-built 2-photon microscope consisting of a Zeiss 410 LSM modified to perform 2-photon excitation laser-scanning microscopy. The microscope was equipped with a titanium-sapphire femtosecond laser (Tsunami; Spectra Physics) tuned and mode locked at 750 nm. For these experiments, a 40×/0.75 NA water objective was used for sample illumination and fluorescence emission collection. Two-photon fluorescence was detected in a nondescanned configuration using a cooled photomultiplier tube (R6060; Hamamatsu). Separation of the emission signal was performed by use of a 690 nm short pass dichroic mirror in the detection path, followed by a bandpass emission filter centered at the quantum dot emission peak (655 ± 10 mn). During acquisition of images, a depth series with a z-separation of 1 μm was acquired throughout the depth of ECs in the flat aorta preparations. Five regions having a field-of-view of 320 × 320 μm2 per section were sampled, and 2 sections per preparation were imaged in each trial. Image capture of samples, prepared in a standardized way, was done using the same 2-photon settings during each experiment. Fluorescence quantitation of 2-photon emission was performed using Metamorph Software (Molecular Devices). Level of threshold was determined from positive and negative samples to assess the mean pixel intensity and was applied uniformly to all samples. Measurements were done in integrated intensities.
Data analysis
Results were expressed as means plus or minus SD, as indicated in each experiment. Student unpaired t test was used to compare the mean values of thrombus sizes and TF activities between treated mice and control groups. Quantitative fluorescence microscopy of VCAM-1 expression ex vivo was analyzed using one-way analysis of variance test, followed by a Tukey multiple comparison test. P values up to .05 denote a statistically significant difference between groups.
Results
Determination of the antigen specificities of the Ab preparations
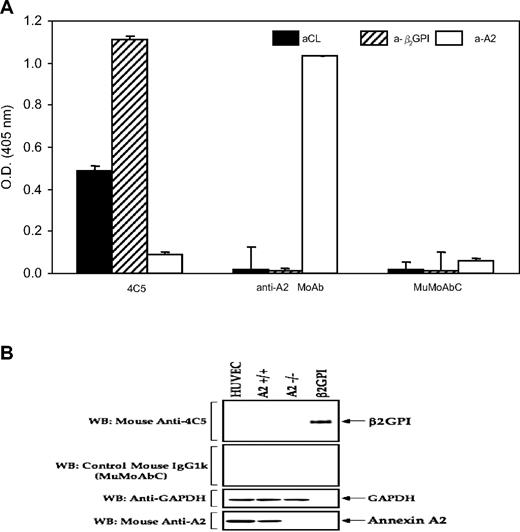
IgG-APS (at 500 μg/mL) had an aCL activity of 178 GPL units/mL and an anti-β2GPI activity of 84 SGU/mL, as determined by ELISA. IgG-APS also showed anti-A2 activity (1.734 O.D. units) by ELISA.
The monoclonal 4C5 had significant titers of β2GPI-dependent aCL and anti-β2GPI Abs (0.488 and 1.109 mean O.D. units, respectively), but not detectable anti-A2 by ELISA (mean 0.094 O.D. units) at 100 μg/mL (Figure 1A). The murine control monoclonal Ab (MuMoAbC) preparation did not have aCL, anti-β2GPI, or anti-A2 activities by ELISA (mean O.D.: 0.02, 0.008, and 0.063, respectively) when tested at a concentration of 100 μg/mL by ELISA. The anti-A2 MoAb preparation did not have β2GPI-dependent aCL or anti-β2GPI Abs by ELISA (0.02 and 0.011 O.D. mean units), but the preparation displayed very high anti-A2 Ab activity (1.034 mean O.D. units). As shown in Figure 1B, 4C5 MoAb displayed anti-β2GPI activity, but not anti-A2 activity, as determined by Western blot analysis, when using purified β2GPI as antigen or A2 from A2+/+ HUVECs, respectively. Lysates of A2−/− HUVECs were used as negative control. Anti-A2 MoAb showed reaction with A2, but not with β2GPI, and the MuMoAbC did not show reactivity with β2GPI or with A2 (Figure 1B), thus confirming the results obtained by ELISA.
Analysis of antigen specificity of the monoclonal Ab preparations. (A) ELISA analysis of antigen specificity. IgG-APS (500 μg/mL), IgG-NHS (500 μg/mL), 4C5 (100 μg/mL), MuMoAbC (100 μg/mL), and anti-A2 MoAb (100 μg/mL) were diluted 1/50 for aCL and 1/100 for anti-β2GPI and anti-A2 assays, and tested by ELISA, as described in “Preparation of immunoglobulin G” and “Western blotting to detect antigen specificity.” Results are expressed as means ± SD O.D. units. (B) Western blot analysis of antigen specificity. Proteins (25 μg/lane from HUVECs, A2+/+, and A2−/−) as well as 200 ng/lane purified β2GPI were resolved on 10% sodium dodecyl sulfate–polyacrylamide gels and blotted with the respective IgGs (4C5 and MuMoAbC). To control for loading, the same blot was stripped and probed with monoclonal IgG directed against A2 and glyceraldehyde-3-phosphate dehydrogenase.
Analysis of antigen specificity of the monoclonal Ab preparations. (A) ELISA analysis of antigen specificity. IgG-APS (500 μg/mL), IgG-NHS (500 μg/mL), 4C5 (100 μg/mL), MuMoAbC (100 μg/mL), and anti-A2 MoAb (100 μg/mL) were diluted 1/50 for aCL and 1/100 for anti-β2GPI and anti-A2 assays, and tested by ELISA, as described in “Preparation of immunoglobulin G” and “Western blotting to detect antigen specificity.” Results are expressed as means ± SD O.D. units. (B) Western blot analysis of antigen specificity. Proteins (25 μg/lane from HUVECs, A2+/+, and A2−/−) as well as 200 ng/lane purified β2GPI were resolved on 10% sodium dodecyl sulfate–polyacrylamide gels and blotted with the respective IgGs (4C5 and MuMoAbC). To control for loading, the same blot was stripped and probed with monoclonal IgG directed against A2 and glyceraldehyde-3-phosphate dehydrogenase.
In vitro studies
Effects of an anti-A2 Ab on IgG-APS–induced expression of E-sel, ICAM-1, and TF activity in HUVECs.
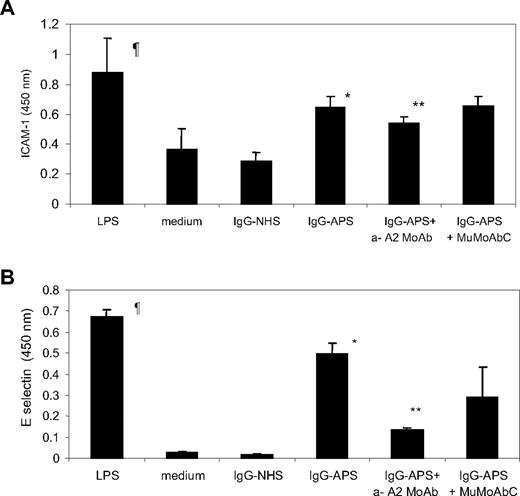
LPS induced a 2- to 3-fold and 20- to 30-fold increase in expression of EC ICAM-1 and E-sel, respectively, compared with that in cells treated with medium alone (Figure 2A-B; P = .001). As demonstrated previously,12,28 the treatment of HUVECs with IgG-APS at a concentration of 200 μg/mL led to a 2- to 3-fold and 20-fold increase in the expression of both cellular adhesion molecules compared with HUVECs treated with either medium or IgG-NHS (Figure 2A-B; P = .001). Pretreatment of the cells with IgG-APS plus anti-A2 MoAb reduced ICAM-1 and E-sel expression by 17% and 69%, respectively (P = .001 for ICAM-1 and .001 for E-sel). Pretreatment of the cells with IgG-APS plus MuMoAbC did not affect the up-regulation of ICAM-1 (P = .865) or E-sel (P = .07) induced by IgG-APS alone.
Effect of anti-A2 on IgG-APS–induced expression of ICAM-1 and E-sel in HUVECs. HUVECs were cultured and treated with either 200 μg/mL IgG-APS or 200 μg/mL IgG-NHS in the presence or absence of 1 μg/mL anti-A2 IgG Ab or 1 μg/mL MuMoAbC, as indicated in “In vitro detection of surface E-sel and ICAM-1 on EC.” Some cells were treated with LPS, as a positive control, or with medium alone, as a negative control. ICAM-1 (A) and E-sel (B) expression were determined by cyto-ELISA, and the data expressed as means ± SD in O.D. units. Experiments were run in triplicate and performed 3 times. ¶Statistically different from medium-treated cells (P = .001). *Statistically different from IgG-NHS–treated cells (P = .001). **Statistically different from IgG-APS–treated cells (ICAM-1, P = .001; E-sel, P = .001).
Effect of anti-A2 on IgG-APS–induced expression of ICAM-1 and E-sel in HUVECs. HUVECs were cultured and treated with either 200 μg/mL IgG-APS or 200 μg/mL IgG-NHS in the presence or absence of 1 μg/mL anti-A2 IgG Ab or 1 μg/mL MuMoAbC, as indicated in “In vitro detection of surface E-sel and ICAM-1 on EC.” Some cells were treated with LPS, as a positive control, or with medium alone, as a negative control. ICAM-1 (A) and E-sel (B) expression were determined by cyto-ELISA, and the data expressed as means ± SD in O.D. units. Experiments were run in triplicate and performed 3 times. ¶Statistically different from medium-treated cells (P = .001). *Statistically different from IgG-NHS–treated cells (P = .001). **Statistically different from IgG-APS–treated cells (ICAM-1, P = .001; E-sel, P = .001).
Cells treated with LPS showed a 1.8-fold increase in TF expression over cells treated with medium alone (Figure 3). Furthermore, treatment of HUVECs with IgG-APS for 4 hours induced significant 2.2-fold increase in TF activity over IgG-NHS–treated cells (P = .01; Figure 3). Importantly, addition of the anti-A2 IgG to IgG-APS reduced 1.4-fold the increase in TF expression (P = .04; Figure 3).
Effect of anti-A2 Ab on TF activity induced by IgG-APS in HUVECs. HUVECs were cultured and treated with either IgG-APS or IgG-NHS in the presence or absence of anti-A2 MoAb, as indicated in “TF functional assay.” Some cells were treated with LPS, as a positive control, or with medium alone, as a negative control. TF activity was determined using a chromogenic assay, and data expressed as fold increase (mean ± SD) over the corresponding control. Experiments were performed 3 times in duplicate. ¶Statistically different from medium-treated cells (P = .031). *Statistically different from IgG-NHS–treated cells (P = .015). **Statistically different from IgG-APS–treated cells (P < .044; P < .05).
Effect of anti-A2 Ab on TF activity induced by IgG-APS in HUVECs. HUVECs were cultured and treated with either IgG-APS or IgG-NHS in the presence or absence of anti-A2 MoAb, as indicated in “TF functional assay.” Some cells were treated with LPS, as a positive control, or with medium alone, as a negative control. TF activity was determined using a chromogenic assay, and data expressed as fold increase (mean ± SD) over the corresponding control. Experiments were performed 3 times in duplicate. ¶Statistically different from medium-treated cells (P = .031). *Statistically different from IgG-NHS–treated cells (P = .015). **Statistically different from IgG-APS–treated cells (P < .044; P < .05).
In vivo studies
Thrombogenic properties of IgG-APS and an anti-β2GPI monoclonal Ab (4C5) are ameliorated in annexin A2−/− mice.
At the time of vascular injury, serum titers of aCL and anti-β2GPI Abs were highly positive (> 80 GPL units) in both A2−/− and A2+/+ mice injected with IgG-APS (99.9 ± 25.9 GPL units and 151.6 ± 16.5 GPL units, respectively). Anti-β2GPI titers were positive in both A2−/− (71.1 ± 30.0 SGU) and A2+/+ mice (52.2 ± 9.7 SGU) injected with IgG-APS. Similarly, all mice injected with 4C5 monoclonal Abs were positive for aCL Abs (O.D. = 0.6 ± 0.1 [A2+/+] or 0.7 ± 0.1 [A2−/−]) and anti-β2GPI Abs (O.D. = 0.8 ± 0.04 [A2+/+] or 1.0 ± 0.1 [A2−/−]), and negative for anti-A2 Abs (O.D. = 0.2 ± 0.14 [A2+/+] or 0.1 ± 0.1 [A2−/−]). Mice injected with anti-A2 MoAb were positive for anti-A2 Abs (O.D. = 1.2 ± 0.2 [A2+/+] or 0.9 ± 0.04 [A2−/−]) and negative for aCL Abs (O.D. = 0.2 ± 0.03 [A2+/+] or 0.1 ± 0.03 [A2−/−]) and anti-β2GPI Abs (O.D. = 0.1 ± 0.0 [A2+/+] or 0.3 ± 0.04 [A2−/−]). All mice injected with MuMoAbC were negative for aCL and for anti-β2GPI Abs and anti-A2 Abs.
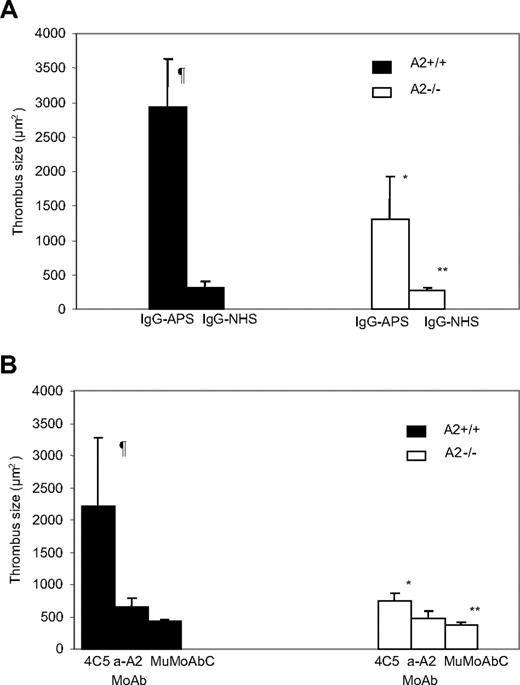
Mean thrombus size was 8 to 9 times larger in A2+/+ mice injected with IgG-APS versus IgG-NHS (P = .001; Figure 4A). Upon IgG-APS injection, mean thrombus size was 60% smaller in A2−/− mice than in A2+/+ mice (P = .002). In A2−/− mice, however, thrombus size was still larger upon injection with IgG-APS versus IgG-NHS (P = .001), indicating a partial, but significant abrogation of the thrombogenic effect in A2−/− mice induced by IgG-APS.
Effect of aPL Abs on thrombus formation in A2−/− mice. A2−/− or A2+/+ mice were treated with either IgG-APS, IgG-NHS (A), 4C5, anti-A2 MoAb, or MuMoAbC as control (B), as indicated in “Analysis of thrombus dynamics.” Thrombi were induced in the animals, and thrombus size was measured in square microns (μm2). The data are expressed as means ± SD (5-10 animals were used per group). ¶Statistically different from A2+/+ mice treated with IgG-NHS (A; P = .001) or MuMoAbC (B; P = .002). *Statistically different from A2+/+ mice treated with IgG-APS (A; P = .002) or 4C5 (B; P = .005). **Statistically different from A2−/− mice treated with IgG-APS (A; P = .001) or 4C5 (B; P = .009).
Effect of aPL Abs on thrombus formation in A2−/− mice. A2−/− or A2+/+ mice were treated with either IgG-APS, IgG-NHS (A), 4C5, anti-A2 MoAb, or MuMoAbC as control (B), as indicated in “Analysis of thrombus dynamics.” Thrombi were induced in the animals, and thrombus size was measured in square microns (μm2). The data are expressed as means ± SD (5-10 animals were used per group). ¶Statistically different from A2+/+ mice treated with IgG-NHS (A; P = .001) or MuMoAbC (B; P = .002). *Statistically different from A2+/+ mice treated with IgG-APS (A; P = .002) or 4C5 (B; P = .005). **Statistically different from A2−/− mice treated with IgG-APS (A; P = .001) or 4C5 (B; P = .009).
To determine whether these in vivo effects were due to aPL Abs (ie, β2GPI-dependent aCL and anti-β2GPI Abs), we carried out experiments using 4C5 MoAb that displayed specificity for β2GPI-dependent aCL and anti-β2GPI activity, but not for anti-A2 Abs, as shown by ELISA and by Western blot. This monoclonal Ab was shown to be thrombogenic and to activate ECs in previous studies.40 Thrombus size was significantly larger in A2+/+ mice injected with 4C5 compared with same type of mice injected with MuMoAbC (P = .002) or with anti-A2 MoAb (P = .017; Figure 4B). The thrombogenic effects of 4C5 were significantly diminished in A2−/− mice (P = .005). In A2−/− mice, however, thrombus size was still larger upon injection with 4C5 compared with MuMoAbC (P = .009), indicating that a partial but significant abrogation of the thrombogenic effect in A2−/− mice induced by 4C5 took place. There was not a statistically significant difference between A2+/+ mice injected with MuMoAbC and with anti-A2 MoAb (P = .681), indicating lack of thrombogenic activity of anti-A2 MoAb alone. There was not a statistically significant difference between A2−/− mice injected with MuMoAbC and with anti-A2 MoAb (P = .299).
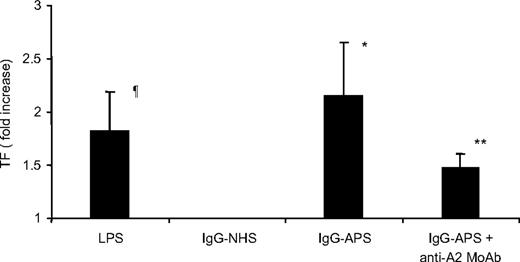
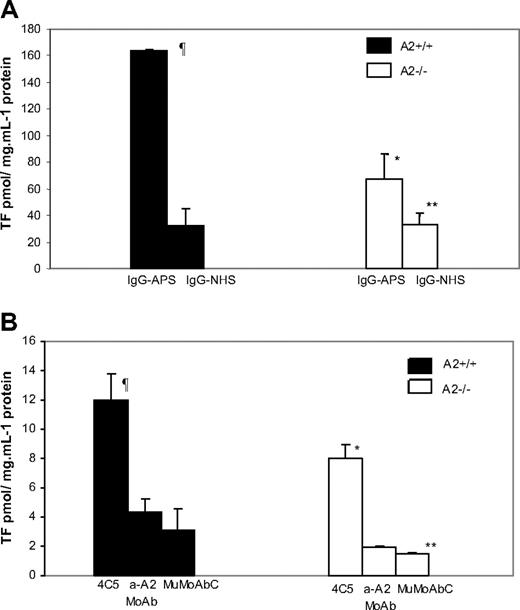
TF activity in carotid artery homogenates was increased 5- to 6-fold in A2+/+ mice treated with IgG-APS versus IgG-NHS (P = .015; Figure 5A). Importantly, upon treatment with IgG-APS, TF activity was diminished by 60% in A2−/− mice compared with their A2+/+ counterparts (P = .026). The absence of A2, however, did not completely eliminate the increase in TF in carotid arteries of mice treated with IgG-APS compared with those treated with IgG-NHS (P = .046). Similarly, TF activity in carotids of A2+/+ mice injected with 4C5 was significantly increased compared with A2+/+ mice injected with MuMoAbC (P = .001) or with anti-A2 MoAb (P = .001). This TF activity was diminished significantly in A2−/− mice treated with 4C5 (P = .007). Again in this case, the up-regulation of TF in carotid arteries in A2−/− mice was significantly elevated compared with A2−/− mice treated with MuMoAbC (P = .002), indicating an incomplete abrogation of the effect of 4C5 on TF in carotids when A2 was absent. There was not a statistically significant difference between A2+/+ mice injected with MuMoAbC and with Anti-A2 MoAb (P = .606; Figure 5B), indicating that anti-A2 MoAb alone do not induce TF up-regulation in carotids of the mice. There was not a statistically significant difference between A2−/− mice injected with MuMoAbC and with anti-A2 MoAb (P = .5; Figure 5B).
Effect of aPL Abs on TF activity in carotid artery homogenates in A2−/− mice. A2−/− or A2+/+ mice were treated with IgG-APS, IgG-NHS (A), 4C5, anti-A2 MoAb, or MuMoAbC as control (B). TF activity was determined in homogenates of carotid arteries using a chromogenic assay, and data expressed as means ± SD in pmol/mg per mL−1 protein. Experiments were assayed in duplicate and repeated thrice. ¶Statistically different from A2+/+ mice treated with IgG-NHS (A; P = .015) or MuMoAbC (B; P = .001). *Statistically different from A2+/+ mice treated with IgG-APS (A; P = .026) or 4C5 (B; P = .007). **Statistically different from A2−/− mice treated with IgG-APS (A; P = .046) or 4C5 (B; P = .002).
Effect of aPL Abs on TF activity in carotid artery homogenates in A2−/− mice. A2−/− or A2+/+ mice were treated with IgG-APS, IgG-NHS (A), 4C5, anti-A2 MoAb, or MuMoAbC as control (B). TF activity was determined in homogenates of carotid arteries using a chromogenic assay, and data expressed as means ± SD in pmol/mg per mL−1 protein. Experiments were assayed in duplicate and repeated thrice. ¶Statistically different from A2+/+ mice treated with IgG-NHS (A; P = .015) or MuMoAbC (B; P = .001). *Statistically different from A2+/+ mice treated with IgG-APS (A; P = .026) or 4C5 (B; P = .007). **Statistically different from A2−/− mice treated with IgG-APS (A; P = .046) or 4C5 (B; P = .002).
Altogether, these data from in vivo experiments in mice reveal that A2 is an important component of the aPL-induced thrombosis and TF up-regulation.
Significant decrease in ex vivo expression of VCAM-1 in A2−/− mice treated with IgG-APS or with a monoclonal anti-β2GPI Ab (4C5)
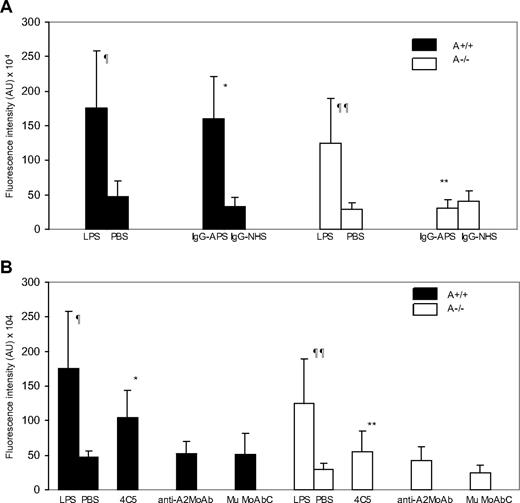
As shown in previous studies,43 IgG-APS induced a 4- to 5-fold up-regulation of VCAM-1 expression in explanted aortas from A2+/+ mice compared with a IgG-NHS control (Figure 6A; 160.3 ± 61.5 × 104 vs 33.4 ± 12.5 × 104 arbitrary units [AU] [shown data as integrated intensity]; P = .001). Because LPS has been shown to up-regulate VCAM-1 expression and this effect is independent of A2, we used LPS-treated mice as positive control. VCAM-1 expression increased 2- to 4-fold in either A2+/+ mice or A2−/− mice treated with LPS, compared with mice treated with PBS (175.8 ± 81.6 × 104 vs 46.4 ± 22.8 × 104 [AU] [P = .001] in A2+/+ mice, and 124.2 ± 65.6 104 vs 50.5 ± 9.0 [AU] [P = .017] in A2−/− mice; Figure 6A-B). There was no difference in LPS-induced VCAM-1 expression in A2+/+ mice compared with A2−/− mice (P = .162), indicating that, as expected, the induction of VCAM-1 expression by LPS was not affected by the lack of A2 in the mice. A2−/− mice treated with IgG-APS showed significantly reduced VCAM-1 expression compared with A2−/− treated with LPS (P = .02). VCAM-1 expression in A2−/− mice treated with IgG-APS was not statistically different from those treated with IgG-NHS or PBS.
Aortic VCAM-1 expression in A2−/− and A2+/+ mice treated with IgG-APS. A2−/− or A2+/+ mice were treated with either IgG-APS, IgG-NHS, LPS, or PBS, as described in “Determination of endothelial VCAM-1 expression in en face preparations of mouse aoura using quautum dot bioconjugates and α-photon excitation laser-scanning microscopy.” VCAM-1 expression was determined by immunostaining using a specific quantum dot bioconjugate, and quantified after examination using a dual photon laser confocal microscope. Fluorescence intensity in AU (mean ± SD; n = 10 images/mouse and 2 mice/group). ¶Statistically different from A2+/+ treated with PBS (P = .001). ¶¶Statistically different from A2−/− treated with PBS (P = .017). *Statistically different from A2+/+ mice treated with IgG-NHS (A; P = .001) or MuMoAbC (B; P = .001). **Statistically different from A2+/+ mice treated with IgG-APS (A; P = .001) or 4C5 (B; P = .017).
Aortic VCAM-1 expression in A2−/− and A2+/+ mice treated with IgG-APS. A2−/− or A2+/+ mice were treated with either IgG-APS, IgG-NHS, LPS, or PBS, as described in “Determination of endothelial VCAM-1 expression in en face preparations of mouse aoura using quautum dot bioconjugates and α-photon excitation laser-scanning microscopy.” VCAM-1 expression was determined by immunostaining using a specific quantum dot bioconjugate, and quantified after examination using a dual photon laser confocal microscope. Fluorescence intensity in AU (mean ± SD; n = 10 images/mouse and 2 mice/group). ¶Statistically different from A2+/+ treated with PBS (P = .001). ¶¶Statistically different from A2−/− treated with PBS (P = .017). *Statistically different from A2+/+ mice treated with IgG-NHS (A; P = .001) or MuMoAbC (B; P = .001). **Statistically different from A2+/+ mice treated with IgG-APS (A; P = .001) or 4C5 (B; P = .017).
Treatment of A2+/+ mice with 4C5 produced a significant increase in VCAM-1 expression ex vivo in the aortas compared with similar type of mice treated with MuMoAbC (P = .001). Up-regulation of VCAM-1 by 4C5 was significantly reduced in A2−/− mice compared with A2+/+ mice (P = .017). Expression of VCAM-1 in aortas of A2+/+ mice treated with anti-A2 MoAb was not different from VCAM-1 expression in A2+/+ mice treated with MuMoAbC (P = .520) or from A2−/− mice treated with anti-A2 MoAb (P = .626; Figure 6B).
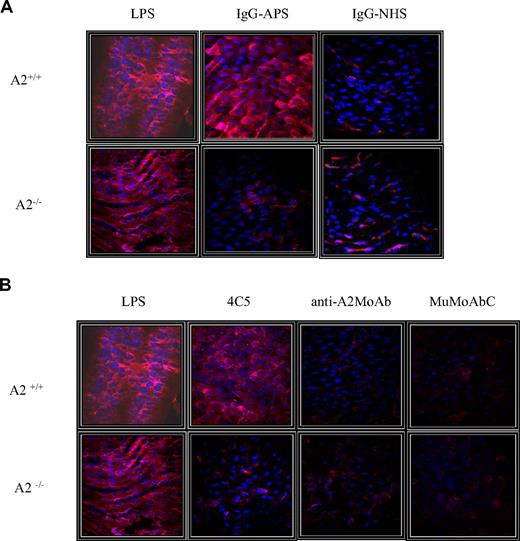
Representative en face images of endothelial VCAM-1 expression of the different treatment groups are shown in Figure 7A and B. The red fluorescent staining (655 nm Qdot bioconjugate) indicates surface VCAM-1 immunoreactivity, whereas the blue color represents EC nuclei stained with Hoechst reagent. Strong signal was observed in aortas of A2+/+ mice treated with IgG-APS or LPS (Figure 7A). A significantly weaker fluorescent signal was imaged for the preparation from A2−/− mice treated with IgG-APS. Similarly, very low fluorescent signal was observed in preparations from mice A2−/− treated with MuMoAbC (Figure 7B). Importantly, aortas of A2+/+ or A2−/− mice injected with anti-A2 monoclonal Ab did not show significant fluorescent signal indicating absence of up-regulation of VCAM-1 expression.
Representative images of endothelial VCAM-1 expression among the different treatment groups. The red fluorescent staining (655 nm Qdot bioconjugate) indicates surface VCAM-1 immunoreactivity, whereas blue staining represents nuclear Hoechst staining. (A) A2+/+ or A2−/− mice were treated with either IgG-APS or IgG-NHS, and LPS or PBS were used as signal controls. (B) A2+/+ or A2−/− mice were treated with 4C5, anti-A2 MoAb, or MuMoAbC as control, and LPS or PBS were used as signal controls.
Representative images of endothelial VCAM-1 expression among the different treatment groups. The red fluorescent staining (655 nm Qdot bioconjugate) indicates surface VCAM-1 immunoreactivity, whereas blue staining represents nuclear Hoechst staining. (A) A2+/+ or A2−/− mice were treated with either IgG-APS or IgG-NHS, and LPS or PBS were used as signal controls. (B) A2+/+ or A2−/− mice were treated with 4C5, anti-A2 MoAb, or MuMoAbC as control, and LPS or PBS were used as signal controls.
Discussion
This study shows for the first time that A2 plays a central role in aPL/anti-β2GPI-pathogenic effects in vivo. APL-induced thrombosis, TF activity, and VCAM-1 expression were all significantly diminished in A2−/− compared with A2+/+ mice. Importantly, the in vivo effects correlated with the inhibitory effect of mouse anti-A2 Ab on up-regulation of ICAM-1, E-sel, and TF induced by aPL Abs in vitro on ECs.
In our study, interestingly, absence of A2 dampened response to IgG-APS on both venous and arterial sides of the circulation. These findings are not surprising as the IgG used was purified from the serum of a patient with both arterial and venous thromboses. Thus, a single Ab may target ECs in both vascular beds through a common mechanism.
Although we recognize the importance of other pathogenic mechanisms induced by aPL Abs, this study specifically addressed effects on EC and thrombosis. Using Western blot analysis and flow cytometry, Zhou et al have shown that both peripheral blood monocytes and the monocytic MM6 cells contained A2 protein in their membrane lysates. These investigators showed further that A2 mediated binding of β2GPI/anti-β2GPI complexes to monocytes and stimulated their expression of TF.51 Cesarman et al reported that Abs directed to A2 are highly prevalent in thrombosis in the setting of APS. They also found that anti-A2 IgG from patients with APS induced TF expression on ECs as effectively as that observed by patient anti-β2GPI Abs.19 In agreement with their findings, the serum used to isolate the IgG-APS and the IgG-APS preparation used in these in vivo and in vitro experiments showed a significant anti-A2 Ab titer. However, in these studies, we conclusively showed, using MoAbs with either anti-β2GPI or anti-A2 activities alone, that the in vivo pathogenic effects (thrombogenic effects and the up-regulation of TF and VCAM-1) were due to the anti-β2GPI and not to the A2 activity of the preparation. These apparent discrepancies between the 2 studies may be due to differences in experimental design or possible different epitopes recognized by the anti-A2 Ab preparations (monoclonal vs polyclonal) in the A2 molecule. It is also possible that patients with APS may have anti-A2 Abs directed to different epitopes that may result in various effects. For example, some Abs may block A2, thus preventing binding of β2GPI (reduced thrombosis), or blocking EC surface fibrinolysis (increasing thrombosis), whereas others may cause a conformational change in A2 that will accelerate binding of β2GPI (increased thrombosis) or initiate a signal itself (increased thrombosis).
In this study, the absence of A2 correlated with a pronounced decrease in up-regulation of VCAM-1 in aortas of A2−/− mice treated with IgG-APS using quantum dot bioconjugates and 2-photon excitation laser-scanning microscopy. This novel en face method improves contrast resolution and allows detailed cellular structures to be imaged without the common problem of vascular autofluorescence. It is, thus, a very promising tool for mapping and quantifying the expression of endothelial markers ex vivo.45,,,,–50
Our in vivo mouse data are in agreement with previous observations that β2GPI binds to A2 on target cells recognized by aPL Abs19,35,36 (Figure 8). Further evidence for the in vitro interaction of β2GPI with anti-A2 Abs and anti-β2GPI was recently provided by Zhou et al, who showed that exogenous β2GPI enhanced the effect of anti-A2 Abs and anti-β2GPI Abs on monocytes and stabilized the formation of a ternary complex, consisting of A2, β2GPI, and the corresponding Ab (anti-A2 or anti-β2GPI).51 In addition, Sorice et al recently showed evidence of anti-β2GPI and A2 interactions in lipid raft fractions of human monocyte plasma membranes.52 In our study, it is noteworthy that the lack of A2 or the addition of an anti-A2 Ab seemed to produce a significant but incomplete inhibition of some of the pathogenic effects induced by aPL Abs both in vitro and in vivo, indicating that when A2 was present, a supplementation effect is observed. Alternatlvely, those findings may indicate that other mechanisms may be involved. For example, recently, Bu et al showed that β2GPI, in addition to binding to A2, is a cofactor for tPA-mediated plasminogen activator.53 Importantly, the authors demonstrated that stimulation of tPA-mediated plasminogen activity by β2GPI was inhibited by a monoclonal anti-β2GPI Ab as well as by anti-β2GPI Abs from patients with APS, suggesting that aPL Abs affect fibrinolysis.53 Alternatively, the partial inhibition in the absence of A2 observed in our in vivo experiments may be due to A2-independent pathogenic mechanisms induced by aPL Abs that are not detected by the methods used in this study. For example, and as discussed below, molecules other than A2 may be involved in binding of β2GPI on various types of target cells (ie, apolipoprotein E receptor 2, Toll-like receptor [TLR]-4, etc), as illustrated in Figure 8.
Diagrammatic representation of interaction of aPL/anti-β2GPI Abs with β2GPI and receptor(s) on ECs. aPL/anti-β2GPI Abs bind to domain I (DI) of β2GPI. β2GPI anchors to annexin A2 on the surface of ECs, possibly through domain V (DV) of the protein. Annexin A2 does not have a transmembrane domain. Hence, it is not able to transduce intracellular signaling. Other membrane proteins (ie, TLR-4 or apoER2′) may act as accessory molecules or may bind β2GPI directly and induce phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) and translocation of nuclear factor-κB (NF-κB), leading to a proinflammatory/prothrombotic phenotype (up-regulation of tissue factor and cellular adhesion molecules [ie, E-sel, ICAM-1, VCAM-1]). Reprinted from Trends in Immunology, P. Meroni, N. Rhonda, E. Raschi, and M.O. Borghi, Humoral immunity against endothelium; theory or reality? 2005;26:275-281, with permission from Elsevier.
Diagrammatic representation of interaction of aPL/anti-β2GPI Abs with β2GPI and receptor(s) on ECs. aPL/anti-β2GPI Abs bind to domain I (DI) of β2GPI. β2GPI anchors to annexin A2 on the surface of ECs, possibly through domain V (DV) of the protein. Annexin A2 does not have a transmembrane domain. Hence, it is not able to transduce intracellular signaling. Other membrane proteins (ie, TLR-4 or apoER2′) may act as accessory molecules or may bind β2GPI directly and induce phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) and translocation of nuclear factor-κB (NF-κB), leading to a proinflammatory/prothrombotic phenotype (up-regulation of tissue factor and cellular adhesion molecules [ie, E-sel, ICAM-1, VCAM-1]). Reprinted from Trends in Immunology, P. Meroni, N. Rhonda, E. Raschi, and M.O. Borghi, Humoral immunity against endothelium; theory or reality? 2005;26:275-281, with permission from Elsevier.
The mechanism by which A2 cross-linking or clustering might mediate anti-β2GPI Ab-induced signal transduction remains unexplained, as A2 is not a transmembrane protein (Figure 8). It has been proposed that activation of signaling responses may require recruitment of another transmembrane adapter protein(s) that associates with A2 on the EC surface. In this regard, Raschi et al previously showed that myeloid differentiation factor 88, an adaptor molecule for TLR-4 that is used to transduce TLR-mediated intracellular signaling, is triggered by aPL/anti-β2GPI Abs on human EC in vitro54 (Figure 8). Subsequently, Zhang et al were able to identify an 83-kD protein that appeared to be TLR-4 among those that bound immobilized β2GPI by affinity purification.55 Sorice et al recently demonstrated the involvement of TLR-4 and A2 as a receptor for aPL/anti-β2GPI Abs in monocyte cell surface lipid rafts.52 This study suggested that the association between β2GPI and TLR-4 was partially dependent on raft integrity. Furthermore, treatment of the cells with anti-β2GPI Abs induced interleukin-1 receptor-associated kinase phosphorylation and subsequent nuclear factor-κB activation, which led to the release of tumor necrosis factor-α and TF.52
Finally, our group carried out experiments in LPS-nonresponsive (LPS−/−) and LPS-responsive (LPS+/+) mice.56 LPS−/− mice display a point mutation of the tlr4 gene leading to the expression of a TLR-4, which does not recognize LPS. IgG isolated from APS patients (IgG-APS; n = 2) produced significantly larger thrombi, induced higher TF activity in carotid artery homogenates, and recruited increased numbers of adhering leukocytes to EC in the microcirculation of the cremaster muscle. This result indicated in vivo activation of EC in LPS+/+ mice treated with IgG-APS compared with IgG-NHS.56 This effect was due to the anti-β2GPI activity present in the IgG fractions, because it was abrogated after removal of such activity from the aPL preparations. The 2 IgG-APS induced significantly smaller thrombus size, lower number of leukocytes adhering to EC, and TF activity in LPS−/− compared with LPS+/+ mice (Figure 8).
In addition, it is possible that apolipoprotein E receptor 2 (apoER2), as shown in platelets, may also be a receptor for β2GPI in target cells57 (Figure 8). Because apoER2 is found in many other cell types, it is possible that apoER2 may also be a receptor for β2GPI in EC and monocytes.58 Taken together, the data suggest that β2GPI binds to A2 on EC and monocytes, and uses TLR-4 and possibly other, as yet unidentified, proteins as coreceptor(s) (Figure 8). These interactions are necessary to trigger intracellular signaling and to induce a proinflammatory/prothrombotic phenotype in monocytes and EC, after cross-linking with specific aPL/anti-β2GPI Abs (Figure 8).
It has been reported that aPL Abs may increase the threshold activation in EC, monocytes, and platelets (“first hit”), whereas a second event or “hit” (infection, trauma, surgery, etc) triggers clinical thrombosis.59 Current treatments in APS are directed at the “second hit” (thrombosis), and include aggressive anticoagulation and immunosuppression, both of which are associated with significant side effects. Treatments that modulate the early effects of aPL Abs on EC would be more beneficial and potentially less harmful than what is currently used. Hence, agents that inhibit the binding of β2GPI to the receptor or the binding of aPL/anti-β2GPI Abs to the β2GPI may directly prevent the first hit, reducing the risk of clinical sequel, should a second hit occur. This is even more important, in light of the fact that second hits (such as common infectious processes) are not easily prevented. Knowing the molecular interactions induced by aPL Abs, the nature of the receptor, and its interaction with β2GPI and specific Abs may help to identify new targeted and more specific modalities for treatment and prevention of thrombosis with fewer adverse effects in patients with aPL Abs.
This study provides new insights on the molecular pathogenesis of aPL Abs and confirms the involvement of A2 in APS-mediated thrombosis using in vitro and in vivo models.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are thankful to Dr Tri Te for her editorial work.
This work was partially supported with funds from the Antiphospholipid Standardization Laboratory (University of Texas Medical Branch) and American Heart Association Grant 0855272F. G.M.-M. was sponsored by the National Medical Center, IMSS. E.G.-L. and E.R.-M. are fellows of the Instituto Politecnico Nacional.
Authorship
Contribution: Z.R.-P., M.G.M.-M., T.S., E.P., A.B.D., M.W., and A.T.J. conducted the experiments of this study and participated in calculating and analyzing data; Z.R.-P., K.A.H., and S.S.P. contributed in the writing of the manuscript; and G.V., E.G.-L., E.R.-M., K.A.H., and S.S.P. participated in the design of the experiments, discussion and analysis of the data, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Silvia S. Pierangeli, Division of Rheumatology, Department of Internal Medicine, 301 University Blvd, Galveston, TX 77555-1165; e-mail: sspieran@utmb.edu.

![Figure 8. Diagrammatic representation of interaction of aPL/anti-β2GPI Abs with β2GPI and receptor(s) on ECs. aPL/anti-β2GPI Abs bind to domain I (DI) of β2GPI. β2GPI anchors to annexin A2 on the surface of ECs, possibly through domain V (DV) of the protein. Annexin A2 does not have a transmembrane domain. Hence, it is not able to transduce intracellular signaling. Other membrane proteins (ie, TLR-4 or apoER2′) may act as accessory molecules or may bind β2GPI directly and induce phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) and translocation of nuclear factor-κB (NF-κB), leading to a proinflammatory/prothrombotic phenotype (up-regulation of tissue factor and cellular adhesion molecules [ie, E-sel, ICAM-1, VCAM-1]). Reprinted from Trends in Immunology, P. Meroni, N. Rhonda, E. Raschi, and M.O. Borghi, Humoral immunity against endothelium; theory or reality? 2005;26:275-281, with permission from Elsevier.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/14/10.1182_blood-2008-11-188698/5/m_zh89990941890008.jpeg?Expires=1765932519&Signature=EG4CgJc1bkQeXryYQFn8c08SKT0rp1gnUjoJ47d8iNshFeuQ~4zHDoNBRwmbq6Y39uOS6gSpfn-rAjwN1A-kdjk6kgz9hMsg85wqneNp3uiWrADfqvCEA4UxWwkrdtFn8ocF9C~HQFmSd8q2uqTxfHxrUZ86nFvKYHJL6Cl4GEQ2AUlyQN2NhTnw1xxZXydEGrEGb4PVLg-6oV8WiA05R2s999sz9o0FLeElDQcWVnu276BQRhshPdjwrwg0~h3OoQAd6-QJTTg6x-pIQkjcc49Kah-BBPZvPxUb9nSzBABZeXQVy4eEHrl957qTfiMIfVAaxV5Ul3HuKt~08t0QBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)