A variety of nonmalignant cells present in the tumor microenvironment promotes tumorigenesis by stimulating tumor cell growth and metastasis or suppressing host immunity. The role of such stromal cells in T-cell lymphoproliferative disorders is incompletely understood. Monocyte-derived cells (MDCs), including professional antigen-presenting cells such as dendritic cells (DCs), play a central role in T-cell biology. Here, we provide evidence that monocytes promote the survival of malignant T cells and demonstrate that MDCs are abundant within the tumor microenvironment of T cell–derived lymphomas. Malignant T cells were observed to remain viable during in vitro culture with autologous monocytes, but cell death was significantly increased after monocyte depletion. Furthermore, monocytes prevent the induction of cell death in T-cell lymphoma lines in response to either serum starvation or doxorubicin, and promote the engraftment of these cells in nonobese diabetic/severe combined immunodeficient mice. Monocytes are actively recruited to the tumor microenvironment by CCL5 (RANTES), where their differentiation into mature DCs is impaired by tumor-derived interleukin-10. Collectively, the data presented demonstrate a previously undescribed role for monocytes in T-cell lymphoproliferative disorders.
Introduction
Stromal cells present in the tumor microenvironment play a central role in tumor cell growth both directly and indirectly via the suppression of host immunity.1,2 Gene-expression profiling analyses in both follicular and diffuse large B-cell non-Hodgkin lymphoma (NHL) illustrate this point, as gene signatures that are of prognostic significance do not necessarily reflect gene expression in tumor cells themselves, but reflect those present in nonmalignant stromal cells, including tumor-infiltrating lymphocytes and monocyte-derived antigen-presenting cells.3,4 Not surprisingly then, cells present in the tumor microenvironment are required to sustain the growth and survival of malignant B cells and may be of greater prognostic significance than conventional tumor characteristics.5 Chemokines and other tumor-associated factors may promote the migration of these cells to the tumor6,–8 ; alternatively, these cells may be generated de novo within the tumor microenvironment.9 Identification of such stromal cells present in the tumor microenvironment has led to the development of novel therapeutic strategies and improved outcomes in human cancers.10,,,,–15 Although the microenvironment's role is increasingly appreciated in B cell–derived NHL, relatively little is known about the tumor microenvironment in T-cell lymphoproliferative disorders.3,4,16,17 As monocyte-derived antigen-presenting cells (eg, dendritic cells) are vitally important in the life cycle of normal T cells, regulating diverse processes ranging from thymic selection and peripheral homeostasis to T-cell activation and differentiation,18 we examined the role of monocytes in mature (postthymic) T-cell lymphoproliferative disorders, including cutaneous T-cell NHL (CTCL) and peripheral T-cell NHL (PTCL).
A growing body of evidence demonstrates that malignant T cells, like their normal counterparts, may be dependent upon monocyte-derived cells (MDCs), including dendritic cells (DCs), for their growth and survival. For example, engagement of the T-cell costimulatory receptor CD28 by its ligands, normally expressed by MDCs, stimulates the proliferation of malignant T cells.19 Similarly, prolonged survival of malignant T cells during in vitro culture has been observed when DCs are included in these cultures.20 Just as DCs influence the differentiation of normal T cells after antigenic stimulation, they are capable of influencing the differentiation program of malignant T cells via the up-regulation of the transcription factor FoxP3.21 As professional antigen-presenting cells at inflammatory or malignant sites are largely derived from monocyte progenitors, we hypothesized that monocytes may be actively recruited to the tumor microenvironment and promote the survival of malignant T cells.
Herein, we demonstrate that monocytes, recruited to the microenvironment by tumor-derived CCL5, promote tumor cell growth and survival. In turn, malignant T cells inhibit the functional maturation of monocyte-derived DCs in an interleukin-10 (IL-10)–dependent manner. Consequently, DCs in T-cell lymphoproliferative disorders may be unable to promote the generation of host antitumor immunity, despite their relative abundance in the tumor microenvironment. Thus, we have identified a previously undescribed role for monocytes in T-cell lymphoproliferative disorders. Targeting monocytes and the cytokines that regulate their recruitment into the tumor microenvironment and inhibit their maturation into fully competent DCs represent novel therapeutic strategies in T-cell lymphoproliferative disorders.
Methods
Cell lines and proliferation assay
The CTCL cell lines SeAx and MyLa (both a generous gift from Dr Robert Gniadecki, University of Copenhagen, Bispebjerg Hospital) were grown in RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (FBS; HyClone); HuT 78 cells (ATCC) were grown in Iscove modified Dulbecco medium supplemented with 20% FBS. The PTCL cell lines SU-DHL-1 (DSMZ) and Karpas 299 (ATCC) were grown in RPMI 1640 (Gibco) supplemented with 10% FBS; SR-786 cells (DSMZ) were grown in 15% FBS. All cells were maintained at 37°C in 5% CO2. All cell lines were mycoplasma free. All cell culture reagents were endotoxin free. Cell line conditioned media were obtained by plating 106 cells in 2 mL complete RPMI in each well of a 24-well plate. After 48 hours, media were centrifuged and cell-free supernatants collected for use. For determination of cell proliferation, 104 cells/well in a 96-well plate were pulsed with 1 μCi (0.037 MBq)/well 3H-tritium deoxyribonucleotide for the last 12 to 15 hours of a 72-hour culture before determination of thymidine incorporation.
Antibodies and flow cytometry
Unless otherwise indicated, fluorochrome-conjugated antibodies used for flow cytometry were obtained from BD Biosciences. Cells were analyzed on a FACSCalibur instrument (Becton Dickinson) and analyzed using CellQuest or FACSDiva Software (Becton Dickinson).
Mice
Nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice were obtained from The Jackson Laboratory and were maintained in a barrier facility in compliance with the Mayo Clinic Institutional Animal Care and Use Committee. For the establishment of tumor xenografts, 6 to 7.5 × 106 MyLa cells with or without an equal number of freshly purified human monocytes were injected subcutaneously in 100 μL phosphate-buffered saline.
Patient samples
Malignant T cells were obtained from peripheral blood and skin biopsy specimens. Malignant T cells were obtained from skin biopsies, as previously described.22 Briefly, autoclaved 9 mm × 9 mm × 1.5 mm Cellfoam matrices (Cellsciences Pte Ltd) were incubated with 100 μg/mL rat tail collagen (BD Biosciences) in phosphate-buffered saline for 30 minutes at 37°C. Matrices were subsequently rinsed before being loaded with minced skin explants. Skin explants were cultured in 24-well plates in Iscove modified medium (Gibco) supplemented with 20% FBS, penicillin and streptomycin, and 3.5 μL/L B-mercaptoethanol. Fresh media were applied 3 times weekly and skin resident T cells harvested 2 to 3 weeks later. For determination of apoptosis, 1 to 2 × 106 patient peripheral blood mononuclear cells (PBMCs) or cutaneous T cells were incubated in each well of a 24- or 48-well plate in either serum-free or complete RPMI 1640. Freshly isolated monocytes (106) or monocyte-conditioned media were included in some experiments. Apoptosis was determined by annexin V (Caltag), and propidium iodide (Sigma) or 7-amino-actinomycin D (7-AAD; EMD Biosciences) costaining, per the manufacturer's instructions. Malignant T cells were identified by the expression of the relevant TCR Vβ chain (Beckman Coulter). In cases where TCR Vβ use was unknown, malignant T cells were identified as CD4+CD7− cells, as these cells may lose the expression of T-cell markers, including CD7.23 The aberrant loss of CD7 expression was confirmed at the time of diagnosis for all patients whose specimens were used in the current study. Apoptosis of malignant T cells was identified by gating on TCR Vβ+ or CD4+CD7− events. Where indicated, relative viability was reported. The relative viability was calculated by dividing the percentage of viable T cells observed in the experimental group by the percentage of viable T cells observed in the mock-depleted (control) group. Patient-derived T cells were cultured in 24-well plates (4 × 106/well) in the presence of PMA (1 μg/mL; Sigma), ionomycin (1 nM; EMD Biosciences), and brefeldin A (supplied as 1000× solution; BioLegend) for 4 to 6 hours and intracellular staining for IL-10 was performed, per the manufacturer's recommendations. Frozen plasma samples from both normal donors and patients were obtained from the Lymphoma SPORE Biospecimens Core Facility. Plasma CCL5 was measured by enzyme-linked immunosorbent assay (kit obtained from PeproTech). Plasma IL-10 was measured by a bead-based human cytokine multiplex assay, per the manufacturer's recommendations (Biosource). All functional studies were performed using fresh material obtained from patients who had provided written informed consent in accordance with the Declaration of Helsinki and were approved by the Institutional Review Board of the Mayo Clinic.
Cell isolation and purification
Monocytes were enriched by negative selection from apheresis cones using a monocyte enrichment kit (StemCell Technologies) and subsequently positively selected using RoboSep (StemCell Technologies), as per the manufacturer's instructions. Purity, as assessed by CD14 staining by flow cytometry, was at least 95%. Monocytes thus isolated were immunophenotyped and found to be CD14+CD11b+CD11c+HLA-DR+CD16−/+ and CD1c−CD206− (data not shown). Purified monocytes retained expression of monocyte markers (eg, CD14) after culture in media alone and when cocultured with malignant T cells (data not shown). Isolated monocytes were able to differentiate into macrophages or DCs when cultured in the appropriate conditions and were responsive to Toll-like receptor agonists (data not shown). CD4+ T cells and CD19+ B cells were isolated in a similar fashion, by either negative or positive selection using RoboSep (StemCell Technologies) and were at least 90% pure. For monocyte depletion experiments, more than 85% of monocytes were removed from peripheral blood mononuclear cells by positive selection of CD14+ monocytes using RoboSep (StemCell Technologies). Mock-depleted cells were obtained in an identical fashion, only cells were incubated with phosphate-buffered saline instead of the positive selection cocktail provided by the manufacturer. Monocyte-conditioned media were obtained by plating 2 × 106 monocytes in 2 mL serum-free RPMI 1640 in a flat-bottom 24-well plate for 48 hours. At least 90% of monocytes remained viable, as determined by trypan blue exclusion, after culture. Supernatants were centrifuged before storage to remove any cell debris and stored at 4°C for up to 1 week for later use. T cell– and B cell–conditioned media were similarly obtained.
Dendritic cells
Purified monocytes (2 × 106/mL) were cultured in 6-well plates in 3 mL total volume of complete RPMI supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF, 100 μg/mL) and IL-4 (10 μg/mL). Fresh media were added every 48 to 72 hours and nonadherent DCs were collected for use on days 6 to 7 of culture. To obtain mature DCs, 1 μg/mL lipopolysaccharide (LPS; Sigma) was added for the final 24 hours of culture. Dendritic cell maturation in response to LPS was verified by the increased expression of maturation markers, including HLA-DR, CD83, CD80, and CD86 (data not shown). In some experiments, 5 × 105 normal T cells or cell lines were added to the DCs for the last 48 hours of culture. CD11c+ DCs were subsequently purified using a PE-conjugated CD11c monoclonal antibody (BD Biosciences) and PE microbeads (Miltenyi Biotec). DCs were irradiated (20 Gy) before use in an allogeneic mixed lymphocyte reaction.
Monocyte chemotaxis
Monocytes were labeled with calcein AM (5 μg/mL; Invitrogen) and 106 cells in 100 μL total volume placed in the top chamber of a 5-μm transwell insert (Fisher Scientific) in triplicate and incubated at 37°C for 2 to 4 hours. Complete RPMI or MyLa-conditioned media (600 μL) were placed in the bottom of each well. Either an isotype control or a neutralizing anti-CCL5 monoclonal antibody (1 μg/mL; R&D Systems) was included in each group. Transwells were removed and migrated cells in the lower chamber measured using a multiwell fluorescent plate reader (CytoFluor II; PerSeptive Biosystems). Fluorescently labeled monocytes placed in lower wells were measured and used as the denominator when calculating the percentage of migration.
Immunohistochemistry
Immunohistochemical staining on paraffin-embedded tissue was performed, as previously described.24 The primary antibodies used for staining include CD1a (Novocastra), CD3 (Novocastra), CD68 (clone PGM-1; Dako), S-100 (Dako), CD11c (Novocastra), CCL5 (R&D Systems), pSTAT3 (Santa Cruz Biotechnology Inc), or CD83 (Novocastra). Malignant T cells were visualized by morphology and CD3 staining, whereas MDCs were identified as CD3− and by their characteristic histiocytic morphology. Where indicated, CD68, CD11c, CD1a, or S-100 staining was used to assist in the identification of MDCs. Bone marrow (megakaryocyte staining for CCL5), breast cancer (pSTAT3), normal skin, lymph node, and tonsil tissue were used as positive controls. Slides were viewed with an Olympus BX51 microscope (20× objective) and pictures taken with an Olympus DP71 camera. Olympus BSW with DP Controller software was used for image acquisition and storage.
Statistical methods
Comparisons among groups were evaluated using a Student t test, chi-square, and Wilcoxon rank sum tests. Statistical analyses were performed using JMP6 (SAS) and SigmaPlot (SSI) software, and P values less than .05 were considered statistically significant.
Results
Monocytes promote survival of malignant T cells
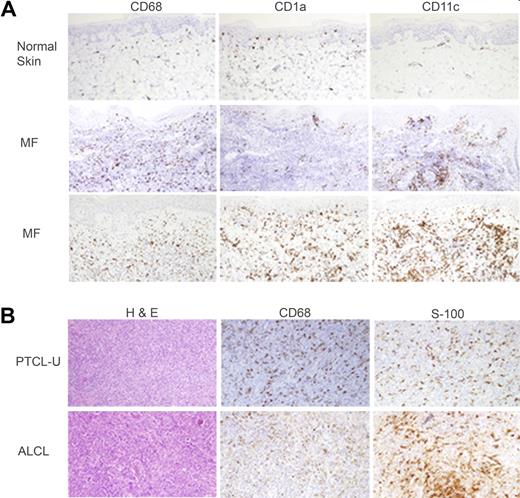
Biopsy samples obtained from both CTCL (n = 10) and PTCL (n = 12) patients were stained for markers expressed by monocyte-derived macrophages or DCs (CD68, CD1a, CD11c, S-100). In the CTCL cases examined, an abundant infiltrate of monocyte-derived cells (MDCs) was easily appreciated compared with normal skin (Figure 1A). Similar results were observed in PTCL (Figure 1B). Given the abundance of MDCs within the tumor microenvironment and the importance of these cells in the biology of normal T cells, we hypothesized that monocytes may promote the survival of malignant T cells. This hypothesis was also motivated by the observation that the absolute number of peripheral blood monocytes is directly proportional to the tumor burden, as reflected in the absolute number of circulating malignant T cells, in selected patients with Sézary syndrome (supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article). For example, the administration of recombinant GM-CSF led to the development of a significant monocytosis that was followed by an approximately 4-fold increase in the patient's tumor burden (supplemental Figure 1B). Upon discontinuation of GM-CSF, the patient's Sézary counts returned to baseline. Not surprisingly, although the patient's monocytes expressed GM-CSF receptor, the patient's malignant T cells did not (supplemental Figure 1C). Furthermore, autologous monocytes isolated from patient peripheral blood mononuclear cells (PBMCs) promote the proliferation of purified malignant T cells (supplemental Figure 1D). Therefore, we sought to explore the possibility that monocytes may promote tumor growth in T-cell NHL.
T-cell lymphoproliferative disorders are associated with a rich infiltrate of monocyte-derived cells. Normal skin and biopsies obtained from CTCL patients with mycosis fungoides (A) or lymph node biopsies from patients with PTCL (B) were stained for monocyte-derived macrophages (CD68) or dendritic cells (CD1a, CD11c, S-100). Representative examples (CTCL, n = 10; PTCL, n = 12) are shown (×200).
T-cell lymphoproliferative disorders are associated with a rich infiltrate of monocyte-derived cells. Normal skin and biopsies obtained from CTCL patients with mycosis fungoides (A) or lymph node biopsies from patients with PTCL (B) were stained for monocyte-derived macrophages (CD68) or dendritic cells (CD1a, CD11c, S-100). Representative examples (CTCL, n = 10; PTCL, n = 12) are shown (×200).
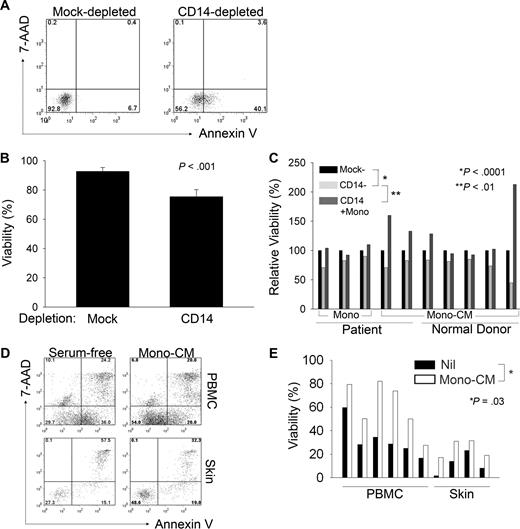
To determine the extent to which monocytes may promote the survival of malignant T cells, monocytes were depleted from PBMCs obtained from T-cell lymphoma patients with circulating malignant cells. As controls, PBMCs were mock depleted, but otherwise handled in an identical fashion. After in vitro culture, the viability of malignant T cells was determined (Figure 2A). An approximately 20% reduction in T-cell viability was observed after monocyte depletion (Figure 2B). We next cocultured monocyte-depleted patient PBMCs with purified autologous monocytes. The subsequent addition of purified monocytes prevented apoptosis in malignant T cells after monocyte depletion (Figure 2C). As the monocytes' ability to promote survival of malignant T cells could be explained by either the provision of costimulatory ligands expressed on the cell surface or cytokine production, we cocultured 3 representative CTCL cell lines with monocytes isolated from the peripheral blood of normal donors. Monocytes were able to promote thymidine incorporation in these cells to a similar degree, whether or not the cells were separated by a transwell insert (supplemental Figure 2A), suggesting that monocytes may prevent apoptosis of malignant T cells via the provision of a soluble factor(s). Similarly, monocyte-depleted PBMCs supplemented with monocyte-conditioned media, whether derived from patient (Figure 2C) or normal donor monocytes (Figure 2C), prevented the induction of apoptosis in malignant T cells after monocyte depletion. Consistent with previous observations,19,20 similar results were obtained using either monocyte-derived dendritic cells or macrophages, suggesting that the production of cytokines that promote the survival of malignant T cells may be a property not entirely specific to monocytes, but one shared among monocytes and their progeny residing in peripheral tissues (data not shown). Therefore, monocytes and MDCs promote the growth and survival of malignant T cells.
Monocytes promote survival of malignant T cells. (A-E) Cells were stained with annexin V and 7-AAD and viability of Sézary cells (SCs) was reported. PBMCs were either mock or CD14 depleted (A-C) and cultured for approximately 7 days before staining. Malignant T cells were identified by staining for the appropriate T-cell receptor (TCR) Vβ chain. For samples in which TCR Vβ use was unknown, malignant CD4+ T cells were identified by the aberrant down-regulation of CD7. The data shown in panel B represent the mean ± SD of 4 independent samples. (C) CD14-depleted samples were supplemented with either purified autologous monocytes (first 3 samples shown, indicated by “mono”) or with supernatants (ie, monocyte-conditioned media; last 7 samples shown, indicated by “Mono-CM”) derived from cultured patient-derived monocytes or with monocyte-conditioned media (mono-CM) derived from normal donor monocytes, as indicated. (D) SCs derived from PBMCs or skin from the same patient were cultured in serum-free media supplemented with autologous mono-CM and viability of CD4+CD7− SCs was determined by annexin V and 7-AAD staining. (E) Total PBMCs (n = 6) or skin-infiltrating lymphocytes (n = 4) were cultured in serum-free (SF) conditions (■) or in SF media supplemented with 40% mono-CM (□) and viability of CD4+CD7− malignant T cells was determined, as described for panel D.
Monocytes promote survival of malignant T cells. (A-E) Cells were stained with annexin V and 7-AAD and viability of Sézary cells (SCs) was reported. PBMCs were either mock or CD14 depleted (A-C) and cultured for approximately 7 days before staining. Malignant T cells were identified by staining for the appropriate T-cell receptor (TCR) Vβ chain. For samples in which TCR Vβ use was unknown, malignant CD4+ T cells were identified by the aberrant down-regulation of CD7. The data shown in panel B represent the mean ± SD of 4 independent samples. (C) CD14-depleted samples were supplemented with either purified autologous monocytes (first 3 samples shown, indicated by “mono”) or with supernatants (ie, monocyte-conditioned media; last 7 samples shown, indicated by “Mono-CM”) derived from cultured patient-derived monocytes or with monocyte-conditioned media (mono-CM) derived from normal donor monocytes, as indicated. (D) SCs derived from PBMCs or skin from the same patient were cultured in serum-free media supplemented with autologous mono-CM and viability of CD4+CD7− SCs was determined by annexin V and 7-AAD staining. (E) Total PBMCs (n = 6) or skin-infiltrating lymphocytes (n = 4) were cultured in serum-free (SF) conditions (■) or in SF media supplemented with 40% mono-CM (□) and viability of CD4+CD7− malignant T cells was determined, as described for panel D.
We next sought to determine whether monocytes may have a similar influence on malignant T cells residing in peripheral extranodal sites. To address this question, malignant T cells were obtained from skin biopsy specimens. Fewer than 30% of malignant T cells, obtained from either PBMCs or skin of the same patient, remained viable when grown in serum-free conditions (Figure 2D). However, culture in media supplemented with autologous monocyte-conditioned media resulted in an approximately 2-fold increase in cell viability (Figure 2D). Similar results were obtained with multiple patient samples (Figure 2E).
As many T-cell lymphoproliferative disorders are characterized by a reactive infiltrate of nonmalignant lymphoid and myeloid cells, we next sought to determine whether T cells or B cells may be able to promote tumor cell survival in a similar fashion. To do so, B cell– and T cell–conditioned media were generated. Whereas monocyte-conditioned media were found to promote thymidine incorporation in T-cell lymphoma lines (Figure 3A and supplemental Figure 2B), conditioned media generated from either B or T cells had no effect. As B cell– and T cell–conditioned media were generated with the same method used to generate monocyte-conditioned media, including exposure to fetal bovine serum during cell isolation, these data exclude the possibility that the effects observed with monocyte-conditioned media are due simply to contamination with fetal bovine serum. Monocytes may play a similar role in T-cell lymphoproliferative disorders of various histologies, as monocyte-conditioned media increased thymidine incorporation in cell lines derived from patients with both CTCL (ie, SeAx, MyLa) and PTCL (ie, SU-DHL-1, Karpas 299; supplemental Figure 2B). The increase in thymidine incorporation observed in response to monocyte-conditioned media is unlikely due to a nonspecific mitogenic effect, as this effect was not observed in all T-cell lymphoma lines (eg, SR-786) tested or in the B-cell lymphoma line DHL6 (data not shown).
Monocytes prevent chemotherapy-induced cell death and promote tumor engraftment in a human xenograft model. (A) HuT 78 cells were cultured in serum-free media alone, in B cell–conditioned media, T cell–conditioned media, patient mono-CM (Mp), or mono-CM obtained from 3 normal donors (Mnd), and thymidine incorporation was determined 72 hours later (mean ± SD). (B) HuT 78 cells in SF or mono-CM were stained with annexin V/PI and viability was determined 48 hours later. (C) HuT 78 cells were cultured in media alone or in media supplemented with mono-CM, and doxorubicin was added at the concentration (80 ng/mL) that inhibited thymidine incorporation at 72 hours by approximately 50%. Data shown are representative of at least 3 independently performed experiments (mean ± SD). (D) MyLa cells, alone or combined with purified monocytes (ratio 1:1), were injected subcutaneously into the flanks of NOD-SCID mice (each group n = 10). Tumors were considered established when the average tumor diameter reached at least 3 mm. Data shown are representative of 4 similarly performed experiments. (E) Tumors were harvested from both groups of mice and immunohistochemistry was performed for markers (CD11c, CD68) expressed by monocyte-derived cells (×200).
Monocytes prevent chemotherapy-induced cell death and promote tumor engraftment in a human xenograft model. (A) HuT 78 cells were cultured in serum-free media alone, in B cell–conditioned media, T cell–conditioned media, patient mono-CM (Mp), or mono-CM obtained from 3 normal donors (Mnd), and thymidine incorporation was determined 72 hours later (mean ± SD). (B) HuT 78 cells in SF or mono-CM were stained with annexin V/PI and viability was determined 48 hours later. (C) HuT 78 cells were cultured in media alone or in media supplemented with mono-CM, and doxorubicin was added at the concentration (80 ng/mL) that inhibited thymidine incorporation at 72 hours by approximately 50%. Data shown are representative of at least 3 independently performed experiments (mean ± SD). (D) MyLa cells, alone or combined with purified monocytes (ratio 1:1), were injected subcutaneously into the flanks of NOD-SCID mice (each group n = 10). Tumors were considered established when the average tumor diameter reached at least 3 mm. Data shown are representative of 4 similarly performed experiments. (E) Tumors were harvested from both groups of mice and immunohistochemistry was performed for markers (CD11c, CD68) expressed by monocyte-derived cells (×200).
In similarly performed experiments, cell viability and growth kinetics were analyzed by trypan blue exclusion (data not shown). This demonstrated that monocyte-conditioned media do not promote cell proliferation to an appreciable degree, but instead may prevent cell death. This was subsequently confirmed, as cell death in response to serum-free conditions was largely prevented upon supplementation of the culture media with monocyte-conditioned media (Figure 3B). Because anthracyclines, such as doxorubicin, are often considered the backbone of multiagent chemotherapy regimens used to treat patients with T-cell lymphoproliferative disorders,25 we cultured HuT 78 cells with doxorubicin at the median effective dose. The presence of monocyte-conditioned media was able to significantly inhibit doxorubicin-induced apoptosis, as measured by either thymidine incorporation (Figure 3C, P < .01) or annexin V/propidium iodide staining (data not shown).
To assess whether monocytes promote tumor growth in vivo, we used a human tumor xenograft model using immunodeficient NOD-SCID mice26 and a CTCL cell line (MyLa). In this model, monocytes were found to promote MyLa cell engraftment. Fewer than 50% of mice injected with MyLa cells alone formed established tumors within 7 days, whereas 90% of mice that were coinjected with freshly purified monocytes formed established tumors (Figure 3D). MDCs were still detectable in these mice up to 3 weeks later, as revealed by immunohistochemical staining for macrophage (CD68) or dendritic cell (CD11c) markers (Figure 3E). As a further control, a cohort of mice received monocytes alone, which did not form tumors or palpable masses (data not shown).
CCL5 recruits monocytes to the tumor microenvironment
The observation that monocytes and their progeny, which are abundant within the tumor microenvironment, promote the growth and survival of malignant T cells suggests that malignant T cells may actively recruit monocytes to the tumor microenvironment. As chemokines play a pivotal role in the trafficking of immune cells, we sought to determine which chemokines may contribute to the migration of monocytes into the tumor microenvironment. Therefore, conditioned media were obtained from T-cell lymphoma cell lines (n = 7) and used to attract normal monocytes in a standard chemotaxis assay. Notably, a positive association was observed between CCL5 levels present in the conditioned media and monocyte migration (R2 = 0.6, P = .04). Culture supernatants from a representative T-cell lymphoma line (MyLa) were used to attract freshly purified monocytes, whereupon CCL5 neutralization was found to abrogate monocyte migration (Figure 4A). Immunohistochemical staining for CCL5 in patient biopsy specimens (n = 10) demonstrated an abundance of CCL5-positive malignant T cells within the tumor microenvironment (Figure 4B). Furthermore, an approximately 5-fold (CTCL) to 10-fold (PTCL) increase in plasma CCL5 was observed in patient plasma compared with normal donors (P < .001; Figure 4C). Therefore, tumor-derived CCL5 may promote monocyte migration into the tumor microenvironment.
CCL5, produced by malignant T cells, attracts monocytes to the tumor microenvironment. (A) MyLa-conditioned media were used to attract monocytes in a 3-hour chemotaxis assay. An isotype control or neutralizing anti-CCL5 antibody was included in the assay, and spontaneous monocyte migration in response to media alone was subtracted from the data shown. Data shown are representative of at least 3 similarly performed experiments (mean ± SD). (B) Skin biopsies from CTCL patients (n = 10) were stained for CCL5. Two representative examples are shown, including an intraepidermal nest of malignant T cells (ie, Pautrier microabscess, indicated by [→]). (C) CCL5 was measured in plasma obtained from both normal donors (n = 24), and from patients with CTCL (n = 23) or PTCL (n = 29). Mean values ± 95% confidence intervals are shown; P < .001.
CCL5, produced by malignant T cells, attracts monocytes to the tumor microenvironment. (A) MyLa-conditioned media were used to attract monocytes in a 3-hour chemotaxis assay. An isotype control or neutralizing anti-CCL5 antibody was included in the assay, and spontaneous monocyte migration in response to media alone was subtracted from the data shown. Data shown are representative of at least 3 similarly performed experiments (mean ± SD). (B) Skin biopsies from CTCL patients (n = 10) were stained for CCL5. Two representative examples are shown, including an intraepidermal nest of malignant T cells (ie, Pautrier microabscess, indicated by [→]). (C) CCL5 was measured in plasma obtained from both normal donors (n = 24), and from patients with CTCL (n = 23) or PTCL (n = 29). Mean values ± 95% confidence intervals are shown; P < .001.
Monocyte differentiation into functionally mature DCs is impaired by tumor-derived IL-10
Monocyte-derived cells, DCs in particular, play an important role in the induction of a fully competent antitumor immune response, depending upon their maturation state.18 As DCs are abundant in the tumor microenvironment and their progenitors are attracted to the tumor site in a CCL5-dependent manner, we sought to determine whether malignant T cells may impair the ability of these cells to become functionally mature, thereby inhibiting their ability to generate an antitumor immune response. Therefore, monocyte-derived DCs were generated and cocultured with a CTCL cell line (HuT 78 cells) before maturation with LPS. Both immature and mature DCs were subsequently isolated and used to stimulate proliferation of allogeneic T cells. As expected, mature DCs (mDCs) were more potent stimulators of allogeneic T-cell proliferation than immature DCs (iDCs). However, the functional maturation of these cells, as demonstrated by their ability to stimulate T-cell proliferation, was greatly impaired when DCs were matured in the presence of HuT 78 cells (Figure 5A). This was not observed in DCs cultured with nonmalignant purified T cells (data not shown). Multiple cytokines, including IL-10 and vascular endothelial-derived growth factor, may impair DC maturation.27,28 As the cell lines used produce IL-1029 and IL-10 (but not vascular endothelial-derived growth factor) was found to be elevated in the plasma of T-cell lymphoma patients30,–32 (data not shown), we next added a neutralizing IL-10 antibody to the DC cocultures, whereupon IL-10 neutralization was found to prevent the functional impairment in DC maturation induced by malignant T cells (Figure 5B-C). Neutralization of IL-10 did not further promote DC maturation in the absence of malignant T cells (data not shown). Consistent with these findings, malignant T cells were found to inhibit the up-regulation of the maturation marker CD83 in an IL-10–dependent manner (Figure 5D). To determine the maturation status of T-cell lymphoma associated DCs, CTCL and benign skin biopsies were immunohistochemically stained for CD83 expression. In benign skin, approximately 10% of CD11c+ DCs expressed CD83; whereas, only trace CD83+ cells were detected in CTCL (Figure 5E). Malignant T cells derived from CTCL patients produce IL-10 (Figure 6A) and an approximately 4-fold increase in plasma IL-10 levels was observed in CTCL patients relative to normal donors (Figure 6B). As IL-10 signaling culminates in the activation of STAT-3, we performed immunohistochemical staining for phosphorylated STAT-3 (pSTAT-3) in CTCL. As shown in Figure 6C, pSTAT3 was appreciated within the tumor microenvironment in all samples analyzed. Therefore, tumor cells not only recruit monocytes into the tumor microenvironment, but they also impair their differentiation into mature DCs in an IL-10–dependent manner, thereby compromising the host's ability to generate a fully competent antitumor immune response.
Malignant T cells inhibit DC maturation in an IL-10–dependent fashion. (A) Immature DCs (iDCs) were generated from monocytes and LPS (1 μg/mL) was added for the last 24 hours of culture to generate mature DCs (mDCs). A group of DCs was cocultured with HuT 78 cells 24 hours before LPS maturation. CD11c+ DCs were purified and iDCs, mDCs, or DCs that had been cocultured with HuT 78 cells before LPS maturation were used to stimulate proliferation of purified CD4+ T cells at the T-cell/DC ratios shown. (B-C) DCs were generated, matured with LPS, and used as stimulators in an allo-mixed lymphocyte reaction at a T-cell/DC ratio of 100:1. As in panel A, before maturation, groups of DCs were cocultured with either HuT 78 (B) or SU-DHL-1 (C) cells in the presence of an isotype control or neutralizing IL-10 monoclonal antibody (2 μg/mL), as indicated. Data are mean ± SD. (D) DCs were generated and matured with LPS in the presence or absence of HuT 78 or SU-DHL-1 cells, as indicated. An isotype control or neutralizing IL-10 antibody was included. Cells were stained with an isotype control (shaded) or anti-CD83 (solid line). Only CD11c+ cells were gated and included in the analysis. Mean fluorescent intensity (MFI) for CD83 is shown in each histogram. Data shown (A-D) are representative of at least 3 similarly performed experiments. (E) Benign dermatitis (n = 10) and CTCL (n = 10) skin biopsies were immunohistochemically stained for both CD11c and CD83. Four representative examples of each are shown.
Malignant T cells inhibit DC maturation in an IL-10–dependent fashion. (A) Immature DCs (iDCs) were generated from monocytes and LPS (1 μg/mL) was added for the last 24 hours of culture to generate mature DCs (mDCs). A group of DCs was cocultured with HuT 78 cells 24 hours before LPS maturation. CD11c+ DCs were purified and iDCs, mDCs, or DCs that had been cocultured with HuT 78 cells before LPS maturation were used to stimulate proliferation of purified CD4+ T cells at the T-cell/DC ratios shown. (B-C) DCs were generated, matured with LPS, and used as stimulators in an allo-mixed lymphocyte reaction at a T-cell/DC ratio of 100:1. As in panel A, before maturation, groups of DCs were cocultured with either HuT 78 (B) or SU-DHL-1 (C) cells in the presence of an isotype control or neutralizing IL-10 monoclonal antibody (2 μg/mL), as indicated. Data are mean ± SD. (D) DCs were generated and matured with LPS in the presence or absence of HuT 78 or SU-DHL-1 cells, as indicated. An isotype control or neutralizing IL-10 antibody was included. Cells were stained with an isotype control (shaded) or anti-CD83 (solid line). Only CD11c+ cells were gated and included in the analysis. Mean fluorescent intensity (MFI) for CD83 is shown in each histogram. Data shown (A-D) are representative of at least 3 similarly performed experiments. (E) Benign dermatitis (n = 10) and CTCL (n = 10) skin biopsies were immunohistochemically stained for both CD11c and CD83. Four representative examples of each are shown.
Malignant T cells produce IL-10 and STAT-3 is phosphorylated within the tumor microenvironment. (A) PBMCs obtained from CTCL patients were cultured in the presence of PMA, ionomycin, and brefeldin A. Malignant T cells were identified as before and intracellular staining for IL-10 was performed. A representative example is shown (n = 2). (B) Plasma was obtained from normal donors (n = 24) and T-cell NHL patients (n = 25) and IL-10 levels were determined (mean ± 95% confidence interval is shown; P = .04). (C) Immunohistochemical analysis of pSTAT3 expression in CTCL biopsy specimens was performed (n = 10). Three representative examples are shown (×200).
Malignant T cells produce IL-10 and STAT-3 is phosphorylated within the tumor microenvironment. (A) PBMCs obtained from CTCL patients were cultured in the presence of PMA, ionomycin, and brefeldin A. Malignant T cells were identified as before and intracellular staining for IL-10 was performed. A representative example is shown (n = 2). (B) Plasma was obtained from normal donors (n = 24) and T-cell NHL patients (n = 25) and IL-10 levels were determined (mean ± 95% confidence interval is shown; P = .04). (C) Immunohistochemical analysis of pSTAT3 expression in CTCL biopsy specimens was performed (n = 10). Three representative examples are shown (×200).
Discussion
Both NHL and Hodgkin lymphoma are composed of both malignant lymphocytes and nonmalignant stromal cells that promote the growth and survival of the malignant cells. Despite the expression of prosurvival genes, such as bcl-2, characteristic of follicular lymphomas, malignant lymphocytes may fail to survive when removed from the microenvironment upon which they depend.33 Furthermore, the abundance of stromal cells appreciated in some lymphomas, such as Hodgkin lymphoma, for example, may not only lead to diagnostic uncertainty, but may also implicate these stromal elements in lymphomagenesis. Interestingly, anecdotal evidence suggests that immunohistochemical staining for markers expressed by MDCs may lead to diagnostic confusion in some patients with T-cell lymphoproliferative disorders34,,,–38 (and data not shown). Therefore, we performed a more systematic analysis for the presence of MDCs in T-cell lymphoproliferative disorders and observed an abundant infiltrate of MDCs (Figures 1 and 5E) in both CTCL and PTCL of various histologies. Furthermore, we observed CD68+ macrophages and CD11c+ dermal DCs within the epidermis of some CTCL patients (Figure 1), suggesting the aberrant localization of these cells within the tumor microenvironment. Given the abundance of MDCs within the tumor microenvironment, we hypothesized that these cells may contribute to the growth and survival of malignant T cells.
Monocyte-derived cells may promote the survival and differentiation status of malignant T cells directly via the provision of both cytokines and T-cell costimulatory ligands.19,–21 For example, previous work has demonstrated that immature DCs may promote the survival of malignant T cells during long-term culture in vitro in a cell-contact–dependent manner.20 We have likewise observed that conditioned media obtained from monocyte-derived macrophages and DCs promote the survival of malignant T cells (data not shown). We have demonstrated that tumor cell death induced by monocyte depletion, culture in serum-free conditions, or doxorubicin was prevented by monocytes or monocyte-conditioned media. Collectively, these data demonstrate that monocytes and their progeny play an important role in promoting the survival of malignant T cells. Of note, a significant reduction in the viability of T cells derived from normal donors was not observed after monocyte depletion. These findings are consistent with the observation that development of peripheral blood monocytosis is an adverse prognostic factor in adult T-cell leukemia/lymphoma, although the prognostic significance of peripheral blood monocytosis has not been evaluated in other T-cell lymphoproliferative disorders.39 To the best of our knowledge, these findings are the first demonstration that monocytes promote malignant T-cell survival, suggesting that the targeting of these cells may represent a novel therapeutic approach in this group of lymphoproliferative disorders for which conventional chemotherapeutic approaches are often unsuccessful.
Whereas some intratumoral immune cells, such as FoxP3+ regulatory T cells (Tregs), may be generated in situ,40 others may be preferentially recruited to the tumor microenvironment by malignant cells. Given the apparent importance of monocytes in T-cell lymphomagenesis and the relative abundance of MDCs within the tumor microenvironment, we examined the possibility that monocytes are directly recruited by malignant T cells. The CCR2-binding chemokines, perhaps most notably CCL2 (MCP-1), play a prominent role in monocyte migration in the setting of infection and inflammation.41 However, CCL2 blockade did not prevent monocyte migration in response to the T-cell lymphoma lines tested, nor was CCL2 elevated in the plasma of patients with T-cell lymphoproliferative disorders (data not shown). In contrast, CCL5 was produced by those T-cell lymphoma lines capable of stimulating monocyte migration, and CCL5 neutralization almost completely inhibited the migration of these cells. CCL5, not typically expressed in normal tissue,42 was produced by malignant T cells in CTCL,43 as revealed by immunohistochemistry, and was elevated in the plasma of such patients. Furthermore, among those patients with progressive disease for whom serial plasma samples were available, CCL5 levels significantly increased during disease progression (data not shown). These findings do not exclude the possibility that nonmalignant cells present in the tumor microenvironment may also contribute to monocyte recruitment44 or that CCL5 may promote the recruitment of additional stromal elements to the tumor microenvironment.8,45 Pharmacologic inhibitors of CCL5 production46 and CCL5 antagonists47,48 have been used in both malignant and inflammatory disorders and may represent an attractive therapeutic approach in T-cell lymphoproliferative disorders.
Tumor-infiltrating DCs, given their ability to activate naive tumor-specific T cells when functionally mature, may promote the generation of a robust antitumor immune response; however, tumor-derived factors may prevent DC maturation,28,48,49 thus impairing host antitumor immunity and promoting tumor-specific tolerance. Given the rich DC infiltrate present within the tumor microenvironment, we speculated that malignant T cells may prevent DC maturation. In contrast to conventional T cells, which may be expected to promote DC maturation via cytokine production and the provision of CD40L, malignant T cells greatly impaired DC maturation in an IL-10–dependent fashion and mature CD83+ DCs were only rarely present in CTCL biopsy specimens. Regulatory T cells are generally understood in relationship to their ability to directly suppress conventional T cells, but recent evidence demonstrates that Tregs may also inhibit T-cell activation indirectly by impairing DC maturation.50,–52 Similarly, malignant T cells may express FoxP3 and directly inhibit the proliferation of conventional T cells, leading some to suggest that particular subsets of T-cell lymphoproliferative disorders may be derived from Tregs. Herein, we provide evidence that malignant T cells may impair T-cell activation indirectly by the inhibition of DC maturation. We also showed that this impairment in DC maturation was IL-10 dependent, as its neutralization reversed the inhibition of CD83 up-regulation on DCs and restored their ability to stimulate the proliferation of conventional T cells. Although the malignant T cells used in these experiments were shown to produce IL-10 by both enzyme-linked immunosorbent assay and intracellular staining (data not shown) and neutralization of IL-10 in DCs cultured alone did not further promote their ability to stimulate T-cell proliferation, we cannot exclude the possibility that DCs may also represent a significant source of IL-10 in T-cell NHL, as IL-10 may be up-regulated by DCs upon engagement with Tregs.51 Cross-talk between malignant T cells on the one hand and nonmalignant T cells and DCs on the other may help to explain the significant immune suppression often observed in patients with T-cell lymphoproliferative disorders.53 Improved understanding of IL-10 regulation, elevated in patients with T-cell lymphoproliferative disorders32 (Figure 6B), and the development of therapeutic strategies capable of either interfering with its production or neutralizing its effects, may represent a useful strategy capable of promoting DC maturation and reversing host immune suppression in these patients.
Collectively, the data presented demonstrate that monocytes and their progeny have a previously unidentified role in T-cell lymphomagenesis—promoting tumor cell growth directly and indirectly, by preventing DC maturation. Targeting monocytes and MDCs in T-cell lymphoproliferative disorders represents a novel therapeutic approach, one potentially capable of both promoting tumor cell death and boosting host immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants CA92104 and CA97274 from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: R.A.W. conceived the study, designed and performed research, analyzed data, and wrote the paper; D.A.W., S.C.Z., and S.F.E. performed research and assisted with data analysis; N.I.C., A.B.D., A.J.N., T.E.W., A.L.F., and M.R.P. analyzed data and critically reviewed the paper; and S.M.A. assisted with research design, data analysis, and paper preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen M. Ansell, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: ansell.stephen@mayo.edu.


![Figure 4. CCL5, produced by malignant T cells, attracts monocytes to the tumor microenvironment. (A) MyLa-conditioned media were used to attract monocytes in a 3-hour chemotaxis assay. An isotype control or neutralizing anti-CCL5 antibody was included in the assay, and spontaneous monocyte migration in response to media alone was subtracted from the data shown. Data shown are representative of at least 3 similarly performed experiments (mean ± SD). (B) Skin biopsies from CTCL patients (n = 10) were stained for CCL5. Two representative examples are shown, including an intraepidermal nest of malignant T cells (ie, Pautrier microabscess, indicated by [→]). (C) CCL5 was measured in plasma obtained from both normal donors (n = 24), and from patients with CTCL (n = 23) or PTCL (n = 29). Mean values ± 95% confidence intervals are shown; P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/14/10.1182_blood-2009-05-220111/5/m_zh89990942990004.jpeg?Expires=1764968695&Signature=YVpCHcl0T9pH674uysd7mCeIFbWKQ5Ty~X~IDpt-Sksuaa663Lyrlnfrgt~DYw7N779ZjZTY4FCM6gAFUlHyCgxnjKUP4Kf~D367AxtR3FJCAy28JhbkwfEtOQjOPk7BEDCcOes0YxnmS9bYWxYJgLntleCGO1RKDsv6OA5q1BgR6~QuohoKaIkjsI3xh9yBdKPQVBZQdhdkdNYIpaYXb~GjjyqC~fnDWej27Jdb10UJUpxKaxNcWM-RJMziciyg9zYgl68~COnn1pemWCejQ1vcCwVRW3CRyip6mwqbzQ7YTsHr1vhlmiwrAoslg6uphNSMldxRfiG8hOnVNPNhIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal